The Lancet Respiratory Medicine ( IF 76.2 ) Pub Date : 2019-09-28 , DOI: 10.1016/s2213-2600(19)30262-0 Luca Richeldi 1 , Evans R Fernández Pérez 2 , Ulrich Costabel 3 , Carlo Albera 4 , David J Lederer 5 , Kevin R Flaherty 6 , Neil Ettinger 7 , Rafael Perez 8 , Mary Beth Scholand 9 , Jonathan Goldin 10 , Kin-Hung Peony Yu 11 , Thomas Neff 11 , Seth Porter 11 , Ming Zhong 11 , Eduard Gorina 11 , Elias Kouchakji 11 , Ganesh Raghu 12
|
Background
Connective tissue growth factor (CTGF) is a secreted glycoprotein that has a central role in the process of fibrosis. This study was designed to assess the safety, tolerability, and efficacy of pamrevlumab (FG-3019), a fully recombinant human monoclonal antibody against CTGF, in idiopathic pulmonary fibrosis. The aim was to establish whether pamrevlumab could slow, stop, or reverse progression of idiopathic pulmonary fibrosis.
Methods
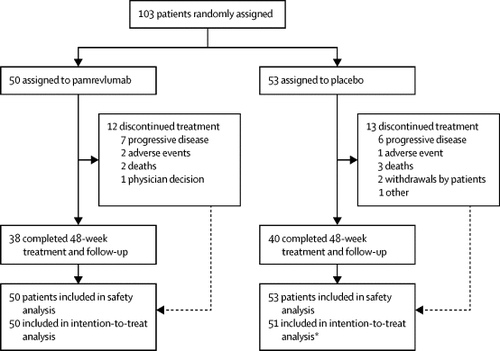
The phase 2, randomised, double-blind, placebo-controlled PRAISE trial was done at 39 medical centres in seven countries (Australia, Bulgaria, Canada, India, New Zealand, South Africa, and the USA). Patients with idiopathic pulmonary fibrosis and percentage of predicted forced vital capacity (FVC) of 55% or greater were enrolled and randomly assigned (1:1) by use of interactive responsive technology to intravenous infusion of pamrevlumab 30 mg/kg or placebo every 3 weeks over 48 weeks (16 infusions). The primary efficacy outcome was change from baseline in percentage of predicted FVC at week 48. Disease progression (defined as a decline from baseline in percentage of predicted FVC of ≥10%, or death) at week 48 was a key secondary efficacy outcome. All patients in the pamrevlumab group received at least one dose of the study drug and were analysed for safety. Two patients in the placebo group were excluded from the intention-to-treat population for the efficacy analyses because of enrolment error. This trial is registered with ClinicalTrials.gov, NCT01890265.
Findings
Between Aug 17, 2013, and July 21, 2017, 103 patients were randomly assigned (50 to pamrevlumab and 53 to placebo). Pamrevlumab reduced the decline in percentage of predicted FVC by 60·3% at week 48 (mean change from baseline −2·9% with pamrevlumab vs −7·2% with placebo; between-group difference 4·3% [95% CI 0·4–8·3]; p=0·033). The proportion of patients with disease progression was lower in the pamrevlumab group than in the placebo group at week 48 (10·0% vs 31·4%; p=0·013). Pamrevlumab was well tolerated, with a safety profile similar to that of placebo. Treatment-emergent serious adverse events were observed in 12 (24%) patients in the pamrevlumab group and eight (15%) in the placebo group, with three patients on pamrevlumab and seven on placebo discontinuing treatment. Of the three (6%) deaths in the pamrevlumab group and six (11%) in the placebo group, none was considered treatment related.
Interpretation
Pamrevlumab attenuated progression of idiopathic pulmonary fibrosis and was well tolerated. Now in phase 3 development, pamrevlumab shows promise as a novel, safe, and effective treatment for idiopathic pulmonary fibrosis.
Funding
FibroGen.
中文翻译:

Pamrevlumab是一种抗结缔组织生长因子疗法,用于特发性肺纤维化(PRAISE):一项2期,随机,双盲,安慰剂对照试验。
背景
结缔组织生长因子(CTGF)是一种分泌的糖蛋白,在纤维化过程中具有重要作用。这项研究旨在评估特发性抗CTGF的完全重组人单克隆抗体pamrevlumab(FG-3019)在特发性肺纤维化中的安全性,耐受性和疗效。目的是确定pamrevlumab是否可以减慢,停止或逆转特发性肺纤维化的进展。
方法
在7个国家(澳大利亚,保加利亚,加拿大,印度,新西兰,南非和美国)的39个医疗中心进行了第2期,随机,双盲,安慰剂对照PRAISE试验。采用互动反应技术,每3周随机使用交互式反应技术,将患有特发性肺纤维化且预期强制肺活量(FVC)百分比为55%或更高的患者纳入研究并随机分配(1:1)超过48周(输注16次)。主要疗效结果是在第48周时相对于基线的预测FVC百分比发生变化。在第48周时,疾病进展(定义为基线时FVC≥10%的百分比下降或死亡)是关键的次要疗效结果。pamrevlumab组的所有患者均接受至少一剂研究药物,并进行了安全性分析。由于入组错误,安慰剂组中的两名患者被排除在意向性治疗人群之外,以进行疗效分析。该试验已在ClinicalTrials.gov(NCT01890265)上注册。
发现
在2013年8月17日至2017年7月21日之间,随机分配了103例患者(其中50例患者接受了pamrevlumab治疗,而53例患者接受了安慰剂治疗)。Pamrevlumab在第48周时将预测FVC的百分比降低了60·3%(pamrevlumab与基线相比的平均变化为−2·9%,而安慰剂为-7.2 %;组间差异为4·3%[95%CI 0·4-8·3]; p = 0·033)。在第48周,帕姆瑞伐单抗组疾病进展患者的比例低于安慰剂组(10·0%vs31·4%;p = 0·013)。Pamrevlumab具有良好的耐受性,其安全性与安慰剂相似。在pamrevlumab组中有12例(24%)患者出现了治疗突发严重不良事件,在安慰剂组中有8例(15%)发生了治疗,其中pamrevlumab组3例患者和安慰剂7例中止治疗。在pamrevlumab组三例(6%)死亡和安慰剂组六例(11%)死亡中,没有一例被认为与治疗有关。
解释
Pamrevlumab减缓了特发性肺纤维化的进展,并且耐受性良好。现在处于3期开发阶段,帕姆瑞夫单抗显示出有望成为一种针对特发性肺纤维化的新颖,安全,有效的治疗方法。
资金
FibroGen。



























 京公网安备 11010802027423号
京公网安备 11010802027423号