Communications Chemistry ( IF 5.9 ) Pub Date : 2019-09-24 , DOI: 10.1038/s42004-019-0210-8 Abbas H. K. Al Temimi , Helene I. V. Amatdjais-Groenen , Y. Vijayendar Reddy , Richard H. Blaauw , Hong Guo , Ping Qian , Jasmin Mecinović
|
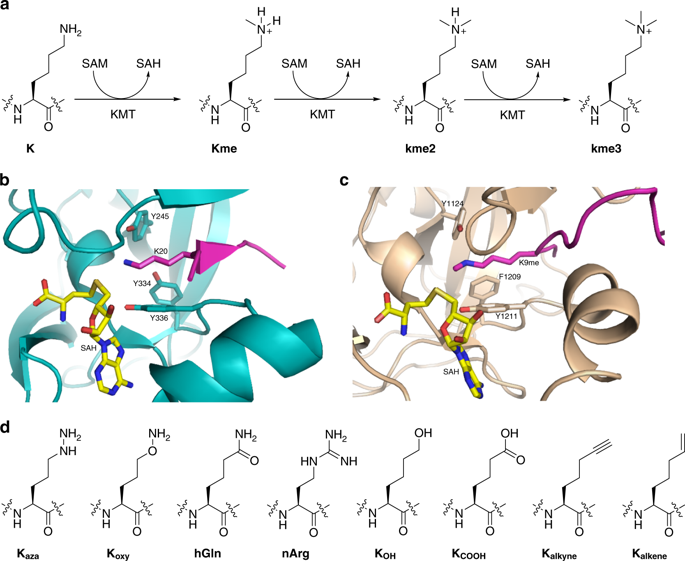
Histone lysine methyltransferases (KMTs) are biomedically important epigenetic enzymes that catalyze the transfer of methyl group from S-adenosylmethionine to lysine’s nucleophilic ε-amino group in histone tails and core histones. Understanding the chemical basis of KMT catalysis is important for discerning its complex biology in disease, structure-function relationship, and for designing specific inhibitors with therapeutic potential. Here we examine histone peptides, which possess simplest lysine analogs with different nucleophilic character, as substrates for human KMTs. Combined MALDI-TOF MS experiments, NMR analyses and molecular dynamics and free-energy simulations based on quantum mechanics/molecular mechanics (QM/MM) potential provide experimental and theoretical evidence that KMTs do have an ability to catalyze methylation of primary amine-containing N-nucleophiles, but do not methylate related amide/guanidine-containing N-nucleophiles as well as simple O- and C-nucleophiles. The results demonstrate a broader, but still limited, substrate scope for KMT catalysis, and contribute to rational design of selective epigenetic inhibitors.
中文翻译:

赖氨酸的亲核氨基是组蛋白赖氨酸甲基转移酶催化的关键
组蛋白赖氨酸甲基转移酶(KMT)是生物医学上重要的表观遗传酶,可催化S上甲基的转移-腺苷甲硫氨酸对组蛋白尾巴和核心组蛋白中赖氨酸的亲核ε-氨基的影响。了解KMT催化的化学基础对于识别其在疾病中的复杂生物学,结构-功能关系以及设计具有治疗潜力的特定抑制剂非常重要。在这里,我们研究了具有最简单的具有不同亲核特征的赖氨酸类似物的组蛋白肽,作为人类KMT的底物。结合MALDI-TOF MS实验,NMR分析以及基于量子力学/分子力学(QM / MM)潜力的分子动力学和自由能模拟,提供了实验和理论证据,表明KMT确实具有催化含伯胺N的甲基化的能力。 -亲核试剂,但不要甲基化相关的含酰胺/胍的N-亲核试剂以及简单的O-和C-亲核试剂。结果表明,KMT催化的底物范围更广,但仍然有限,并且有助于选择性表观遗传抑制剂的合理设计。



























 京公网安备 11010802027423号
京公网安备 11010802027423号