当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of a Split Esterase for Protein–Protein Interaction-Dependent Small-Molecule Activation
ACS Central Science ( IF 18.2 ) Pub Date : 2019-09-24 , DOI: 10.1021/acscentsci.9b00567 Krysten A. Jones 1 , Kaitlin Kentala 1 , Michael W. Beck 1 , Weiwei An 2 , Alexander R. Lippert 2 , Jared C. Lewis 3 , Bryan C. Dickinson 1
ACS Central Science ( IF 18.2 ) Pub Date : 2019-09-24 , DOI: 10.1021/acscentsci.9b00567 Krysten A. Jones 1 , Kaitlin Kentala 1 , Michael W. Beck 1 , Weiwei An 2 , Alexander R. Lippert 2 , Jared C. Lewis 3 , Bryan C. Dickinson 1
Affiliation
|
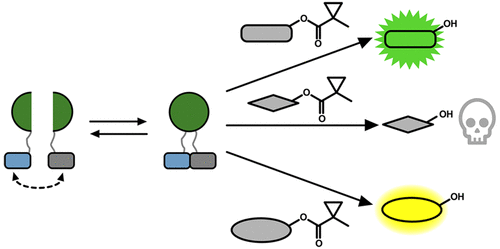
Split reporters based on fluorescent proteins and luciferases have emerged as valuable tools for measuring interactions in biological systems. Relatedly, biosensors that transduce measured input signals into outputs that influence the host system are key components of engineered gene circuits for synthetic biology applications. While small-molecule-based imaging agents are widely used in biological studies, and small-molecule-based drugs and chemical probes can target a range of biological processes, a general method for generating a target small molecule in a biological system based on a measured input signal is lacking. Here, we develop a proximity-dependent split esterase that selectively unmasks ester-protected small molecules in an interaction-dependent manner. Exploiting the versatility of an ester-protected small-molecule output, we demonstrate fluorescent, chemiluminescent, and pharmacological probe generation, each created by masking key alcohol functional groups on a target small molecule. We show that the split esterase system can be used in combination with ester-masked fluorescent or luminescent probes to measure protein–protein interactions and protein–protein interaction inhibitor engagement. We demonstrate that the esterase-based reporter system is compatible with other commonly used split reporter imaging systems for the simultaneous detection of multiple protein–protein interactions. Finally, we develop a system for selective small-molecule-dependent cell killing by unmasking a cytotoxic molecule using an inducible split esterase. Presaging utility in future synthetic biology-based therapeutic applications, we also show that the system can be used for intercellular cell killing via a bystander effect, where one activated cell unmasks a cytotoxic molecule and kills cells physically adjacent to the activated cells. Collectively, this work illustrates that the split esterase system is a valuable new addition to the split protein toolbox, with particularly exciting potential in synthetic biology applications.
中文翻译:

蛋白质-蛋白质相互作用依赖性小分子活化的分裂酯酶的开发
基于荧光蛋白和萤光素酶的拆分报告基因已成为衡量生物系统中相互作用的有价值的工具。相关地,将测得的输入信号转换成影响宿主系统的输出的生物传感器是合成生物学应用中基因工程电路的关键组成部分。尽管基于小分子的成像剂已广泛用于生物学研究中,并且基于小分子的药物和化学探针可以靶向多种生物学过程,但一种通用的方法是根据生物分子的测量结果在生物系统中生成目标小分子缺少输入信号。在这里,我们开发了一种邻近依赖性分裂酯酶,该酶以相互作用依赖性方式选择性地掩盖了酯保护的小分子。利用酯保护的小分子产物的多功能性,我们展示了荧光,化学发光和药理探针的产生,它们各自都是通过掩盖目标小分子上的关键醇官能团而产生的。我们证明了分离酯酶系统可与酯掩蔽的荧光或发光探针结合使用,以测量蛋白质-蛋白质相互作用和蛋白质-蛋白质相互作用抑制剂的参与。我们证明基于酯酶的报道分子系统与其他常用的拆分报道分子成像系统兼容,可同时检测多种蛋白质之间的相互作用。最后,我们开发了一种系统,通过使用诱导分裂酯酶来揭露细胞毒性分子,从而选择性杀死依赖小分子的细胞。预示在未来基于合成生物学的治疗应用中的效用,我们还显示该系统可用于通过旁观者效应杀死细胞间细胞,其中一个激活的细胞可掩盖细胞毒性分子并杀死与激活的细胞物理相邻的细胞。总的来说,这项工作说明了分离酯酶系统是分离蛋白工具箱中有价值的新成员,在合成生物学应用中具有特别令人兴奋的潜力。
更新日期:2019-11-28
中文翻译:

蛋白质-蛋白质相互作用依赖性小分子活化的分裂酯酶的开发
基于荧光蛋白和萤光素酶的拆分报告基因已成为衡量生物系统中相互作用的有价值的工具。相关地,将测得的输入信号转换成影响宿主系统的输出的生物传感器是合成生物学应用中基因工程电路的关键组成部分。尽管基于小分子的成像剂已广泛用于生物学研究中,并且基于小分子的药物和化学探针可以靶向多种生物学过程,但一种通用的方法是根据生物分子的测量结果在生物系统中生成目标小分子缺少输入信号。在这里,我们开发了一种邻近依赖性分裂酯酶,该酶以相互作用依赖性方式选择性地掩盖了酯保护的小分子。利用酯保护的小分子产物的多功能性,我们展示了荧光,化学发光和药理探针的产生,它们各自都是通过掩盖目标小分子上的关键醇官能团而产生的。我们证明了分离酯酶系统可与酯掩蔽的荧光或发光探针结合使用,以测量蛋白质-蛋白质相互作用和蛋白质-蛋白质相互作用抑制剂的参与。我们证明基于酯酶的报道分子系统与其他常用的拆分报道分子成像系统兼容,可同时检测多种蛋白质之间的相互作用。最后,我们开发了一种系统,通过使用诱导分裂酯酶来揭露细胞毒性分子,从而选择性杀死依赖小分子的细胞。预示在未来基于合成生物学的治疗应用中的效用,我们还显示该系统可用于通过旁观者效应杀死细胞间细胞,其中一个激活的细胞可掩盖细胞毒性分子并杀死与激活的细胞物理相邻的细胞。总的来说,这项工作说明了分离酯酶系统是分离蛋白工具箱中有价值的新成员,在合成生物学应用中具有特别令人兴奋的潜力。

























 京公网安备 11010802027423号
京公网安备 11010802027423号