当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Elucidating cation effects in homogeneously catalyzed formic acid dehydrogenation†
Faraday Discussions ( IF 3.4 ) Pub Date : 2019-09-23 , DOI: 10.1039/c9fd00055k Nitish Govindarajan 1, 2, 3, 4, 5 , Evert Jan Meijer 1, 2, 3, 4, 5
Faraday Discussions ( IF 3.4 ) Pub Date : 2019-09-23 , DOI: 10.1039/c9fd00055k Nitish Govindarajan 1, 2, 3, 4, 5 , Evert Jan Meijer 1, 2, 3, 4, 5
Affiliation
|
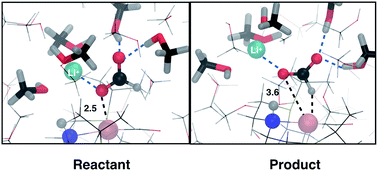
In this work, we use density functional theory based molecular dynamics with an explicit description of methanol solvent to study the effect of cations on formic acid dehydrogenation catalyzed by a ruthenium PNP pincer complex (RuPNP). Formic acid dehydrogenation is a two step process that involves the reorientation of the formate moiety bound via its oxygen to a H bound intermediate, followed by the hydride transfer step to form CO2 and the hydrogenated catalyst. We find the reorientation step to proceed with a low barrier in methanol solvent and in the presence of a Li+ cation, while the hydride transfer is significantly hindered by the presence of cations (Li+ and K+). The cation seems to strongly stabilize the negatively charged formate moiety, hindering complete hydride transfer and resulting in a high barrier for this step. This study is a first step towards addressing the exact role of cations in formic acid dehydrogenation reactions.
中文翻译:

阐明均相催化甲酸脱氢中的阳离子作用†
在这项工作中,我们使用基于密度泛函理论的分子动力学以及对甲醇溶剂的明确描述来研究阳离子对钌PNP夹杂物(RuPNP)催化的甲酸脱氢的影响。甲酸脱氢是两个步骤的过程,涉及将通过其氧结合的甲酸酯部分重新定向到与H结合的中间体,然后进行氢化物转移步骤以形成CO 2和氢化的催化剂。我们发现在甲醇溶剂中和存在Li +阳离子的情况下,低取向势能使重取向步骤得以进行,而阳离子(Li +和K +)。阳离子似乎能强烈稳定带负电荷的甲酸部分,从而阻碍了氢化物的完全转移,并为该步骤带来了高障碍。这项研究是解决阳离子在甲酸脱氢反应中确切作用的第一步。
更新日期:2019-12-04
中文翻译:

阐明均相催化甲酸脱氢中的阳离子作用†
在这项工作中,我们使用基于密度泛函理论的分子动力学以及对甲醇溶剂的明确描述来研究阳离子对钌PNP夹杂物(RuPNP)催化的甲酸脱氢的影响。甲酸脱氢是两个步骤的过程,涉及将通过其氧结合的甲酸酯部分重新定向到与H结合的中间体,然后进行氢化物转移步骤以形成CO 2和氢化的催化剂。我们发现在甲醇溶剂中和存在Li +阳离子的情况下,低取向势能使重取向步骤得以进行,而阳离子(Li +和K +)。阳离子似乎能强烈稳定带负电荷的甲酸部分,从而阻碍了氢化物的完全转移,并为该步骤带来了高障碍。这项研究是解决阳离子在甲酸脱氢反应中确切作用的第一步。



























 京公网安备 11010802027423号
京公网安备 11010802027423号