European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.9 ) Pub Date : 2019-09-18 , DOI: 10.1016/j.ejpb.2019.09.015 Joao A.C. Barbosa , Maha M. Al-Kauraishi , Alan M. Smith , Barbara R. Conway , Hamid A. Merchant
|
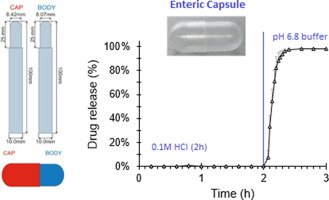
Capsules are a widely used oral dosage form due to their simplicity and ease of manufacture. They are equally popular for both pharmaceutical and nutraceutical products and since they do not need extensive formulation development, it is a dosage form of choice for new drugs undergoing animal or clinical trials. In addition to the standard hard-gelatin or cellulose-based vegetarian capsules, functional capsules such as those with built-in gastroresistance would be of great value. In this work, commonly used enteric polymers were investigated for the production of hard-capsules. The polymers used in this study included cellulose derivatives (HPMC AS-LF and HP-55) and acrylic/methacrylic acid derivatives (EUDRAGIT L100 and S100). A range of concentrations of polymers and plasticisers were tested to optimise the formulation for the production of capsule shells with desirable physicochemical and gastroresistance characteristics. Drug release from optimised capsules produced from HPMC AS-LF, HP-55, EUDRAGIT L100 and S100 was shown to be comparable to drug release from corresponding polymer-coated tablets in both compendial and physiological bicarbonate buffer. In summary, herein we report a simple method for producing enteric capsule shells which do not need an additional coating step which, if validated at large scale, can significantly reduce the cost of manufacturing of conventional enteric coated dosage forms. These capsules are also likely to improve the inter-tablet variability in post-gastric drug release inherent in conventional dosage forms due to coating variability.
中文翻译:

无需包衣即可实现肠胃抵抗性:由肠溶性聚合物制成胶囊壳
胶囊由于其简单性和易制造性而被广泛使用为口服剂型。它们在药品和营养保健产品中同样受欢迎,并且由于不需要大量的制剂开发,因此它是经过动物或临床试验的新药的首选剂型。除了标准的硬明胶或纤维素基素食胶囊外,功能性胶囊(例如具有内置肠胃抵抗性的胶囊)也将具有很大的价值。在这项工作中,对常用的肠溶性聚合物进行了研究以生产硬胶囊。本研究中使用的聚合物包括纤维素衍生物(HPMC AS-LF和HP-55)和丙烯酸/甲基丙烯酸衍生物(EUDRAGIT L100和S100)。测试了一系列浓度的聚合物和增塑剂,以优化配方,以生产具有所需理化和耐胃液特性的胶囊壳。从由HPMC AS-LF,HP-55,EUDRAGIT L100和S100生产的优化胶囊中释放出的药物与在药典和生理性碳酸氢盐缓冲液中从相应的聚合物包衣片剂释放出的药物相当。总而言之,本文中我们报告了一种制备肠溶胶囊壳的简单方法,该方法不需要额外的包衣步骤,如果大规模验证,该步骤可以显着降低常规肠溶包衣剂型的生产成本。



























 京公网安备 11010802027423号
京公网安备 11010802027423号