Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2019-09-16 , DOI: 10.1016/j.jfluchem.2019.109373 Žiga Zupanek , Melita Tramšek , Anton Kokalj , Gašper Tavčar
|
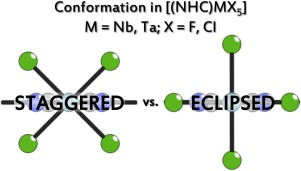
Reaction of niobium or tantalum pentafluoride (MF5; M=Nb, Ta) with N-heterocyclic carbene 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene (LDipp) yields neutral [(LDipp)MF5] (M=Nb (1), Ta (2)) complex. Special attention in their characterization was given to the structural analysis and to the comparison of structural features to the equivalent chloride complexes. Particularly interesting is conformation of the MX5 (M=Nb, Ta; X=F, Cl) unit in relation to the bonded LDipp ligand. In presented compounds (1, 2) the MF5 group is in a staggered position, which concurs with DFT calculations of structurally optimized [(LDipp)NbF5]. In contrast, eclipsed orientation is preferred in equivalent chloride complexes, according to both experiment and DFT calculations. The preference between staggered and eclipsed conformations is attributed to an interplay between attractive C–H⋯X interactions and steric repulsion between equatorial halogen atoms and “wingtips” of the ligand. To help distinguish products detected by NMR spectroscopy, ionic [(LDipp)H][MF6] (M=Nb (3), Ta (4)) compounds were prepared in the reaction between MF5 and “naked” fluoride reagent [(LDipp)H][F]. Disorder in the anionic part of the [(LDipp)H][MF6] prevented unambiguous determination in the crystal structure. Therefore, additional crystallization systems were used and crystal structures of [(LDipp)H][MF6]·2MeCN (M=Nb (3a), Ta (4a)) and [(LDipp)H][MF6]·DCM (M=Nb (3b), Ta (4b)) solvates were obtained in which no disorder was observed.
中文翻译:

V族五卤化物与N-杂环卡宾配位化合物的特殊构象及其咪唑鎓盐的合成
五氟化铌或五氟化钽(MF 5 ; M = Nb,Ta)与N杂环卡宾1,3-双(2,6-二异丙基苯基)咪唑-2-亚烷基(L Dipp)反应生成中性[(L Dipp)MF 5 ](M = Nb(1),Ta(2))络合物。在表征过程中特别注意了结构分析以及与等效氯化物配合物的结构特征比较。MX 5(M = Nb,Ta; X = F,Cl)单元相对于键合的L Dipp配体的构象特别令人感兴趣。中提出的化合物(1,2)的MF 5基团处于交错位置,这与结构优化的[(L Dipp)NbF 5 ]的DFT计算一致。相反,根据实验和DFT计算,在等效的氯化物络合物中,偏光方向是优选的。交错和偏光构型之间的偏爱归因于有吸引力的C–H⋯X相互作用与赤道卤原子与配体的“翼尖”之间的空间排斥之间的相互作用。为了帮助区分通过NMR光谱检测到的产物,在MF 5和“裸”氟化物试剂[[ N]的反应中制备了离子型[[L Dipp)H] [MF 6 ](M = Nb(3),Ta(4))化合物[ (L迪普)H] [F]。[(L Dipp)H] [MF 6 ]的阴离子部分的无序阻止了晶体结构的明确确定。因此,使用了另外的结晶体系,[[(L Dipp)H] [MF 6 ]·2MeCN(M = Nb(3a),Ta(4a))和[(L Dipp)H] [MF 6 ]·的晶体结构被使用。获得了DCM(M = Nb(3b),Ta(4b))溶剂化物,其中未观察到无序。


























 京公网安备 11010802027423号
京公网安备 11010802027423号