Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial.
The Lancet ( IF 168.9 ) Pub Date : 2019-09-15 , DOI: 10.1016/s0140-6736(19)32135-x Rajiv Agarwal 1 , Patrick Rossignol 2 , Alain Romero 3 , Dahlia Garza 4 , Martha R Mayo 4 , Suzette Warren 4 , Jia Ma 5 , William B White 6 , Bryan Williams 7
中文翻译:

Patiromer 与安慰剂相比,使螺内酯能够用于顽固性高血压和慢性肾病 (AMBER) 患者:一项 2 期、随机、双盲、安慰剂对照试验。
更新日期:2019-10-25
The Lancet ( IF 168.9 ) Pub Date : 2019-09-15 , DOI: 10.1016/s0140-6736(19)32135-x Rajiv Agarwal 1 , Patrick Rossignol 2 , Alain Romero 3 , Dahlia Garza 4 , Martha R Mayo 4 , Suzette Warren 4 , Jia Ma 5 , William B White 6 , Bryan Williams 7
Affiliation
|
Background
Spironolactone is effective at reducing blood pressure in patients with uncontrolled resistant hypertension. However, the use of spironolactone in patients with chronic kidney disease can be restricted by hyperkalaemia. We evaluated use of the potassium binder patiromer to allow more persistent use of spironolactone in patients with chronic kidney disease and resistant hypertension.Methods
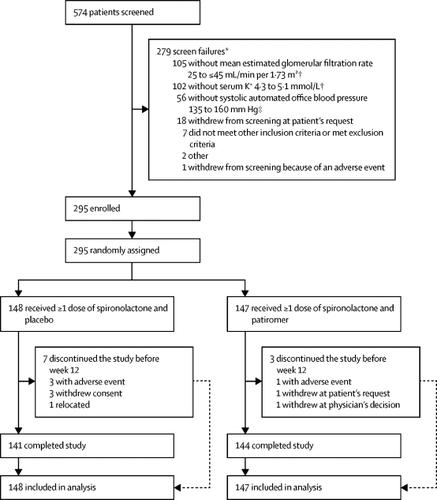
In this phase 2 multicentre, randomised, double-blind, placebo-controlled study, we enrolled participants aged 18 years and older with chronic kidney disease (estimated glomerular filtration rate 25 to ≤45 mL/min per 1·73 m 2) and uncontrolled resistant hypertension from 62 outpatient centres in ten countries (Bulgaria, Croatia, Georgia, Hungary, Ukraine, France, Germany, South Africa, the UK, and the USA). Patients meeting all eligibility criteria at the final screening visit were stratified by local serum potassium measurement (4·3 to <4·7 mmol/L vs 4·7 to 5·1 mmol/L) and history of diabetes. Participants were randomly assigned (1:1) with an interactive web response system to receive either placebo or patiromer (8·4 g once daily), in addition to open-label spironolactone (starting at 25 mg once daily) and their baseline blood pressure medications. Participants, the study team that administered treatments and measured blood pressure, and the investigators were masked to assigned treatment groups. Dose titrations were permitted after 1 week (patiromer) and 3 weeks (spironolactone). The primary endpoint was the between-group difference at week 12 in the proportion of patients on spironolactone. Efficacy endpoints and safety were assessed in all randomised patients (intention to treat). The study was registered with , .Findings
Between Feb 13, 2017, and Aug 20, 2018, we screened 574 patients. 295 (51%) of 574 patients met all inclusion criteria and were randomly assigned to spironolactone in addition to double-blind treatment with either placebo (n=148) or patiromer (n=147). At week 12, 98 (66%) of 148 patients in the placebo group and 126 (86%) of 147 patients in the patiromer group remained on spironolactone (between-group difference 19·5%, 95% CI 10·0–29·0; p<0·0001). Adverse events were mostly mild or moderate in severity and occurred in 79 (53%) of 148 patients in the placebo group and 82 (56%) of 147 patients in the patiromer group.Interpretation
In patients with resistant hypertension and chronic kidney disease, patiromer enabled more patients to continue treatment with spironolactone with less hyperkalaemia. Persistent spironolactone enablement in this population of patients has clinical relevance for the treatment of resistant hypertension.Funding
Relypsa, a Vifor Pharma Group Company.中文翻译:

Patiromer 与安慰剂相比,使螺内酯能够用于顽固性高血压和慢性肾病 (AMBER) 患者:一项 2 期、随机、双盲、安慰剂对照试验。



























 京公网安备 11010802027423号
京公网安备 11010802027423号