European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.9 ) Pub Date : 2019-09-14 , DOI: 10.1016/j.ejpb.2019.09.012 Christina Leichner , Max Jelkmann , Felix Prüfert , Flavia Laffleur , Andreas Bernkop-Schnürch
|
Aim
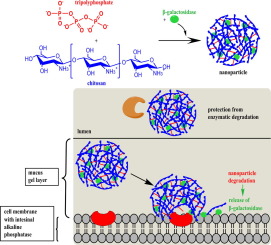
The aim of this study was to evaluate the potential of chitosan/tripolyphosphate (TPP) nanoparticles to provide a targeted release of β-galactosidase behind the intestinal mucus gel barrier.
Methods
Nanoparticles were prepared by ionic gelation of chitosan and TPP in the presence of β-galactosidase. Particles were characterized regarding size, polydispersity index and drug load. Target mediated hydrolysis of the TPP cross-linker followed by particle degradation and release of β-galactosidase was investigated during incubation with isolated as well as cell and tissue associated intestinal alkaline phosphatase (IAP). Phosphate content in the media was quantified via malachite assay, whereas particle disintegration was monitored in parallel by measuring the decrease in particle size as well as in optical density at 600 nm. The released amount of β-galactosidase was either determined utilizing bicinchoninic acid (BCA) protein detection or via an enzymatic activity assay with 2-nitrophenyl β-D-galactopyranoside (ONPG) as substrate. Protection towards tryptic degradation was verified by ONPG assay.
Results
The size of nanoparticles was 573 ± 34 nm and a payload of 376 ± 18 µg β-galactosidase per mg particles was achieved. Degradation studies with isolated IAP revealed a maximum phosphate cleavage of 118 ± 1 µg/mg particles, a size decrease up to 38 ± 7 % and a release of 58 ± 0.5 % β-galactosidase. Release of 94 ± 6 % of the incorporated initial amount of β-galactosidase was proven after 3 h incubation on porcine mucosa. Furthermore a protection against tryptic degradation was attained resulting in a 3-fold higher residual enzymatic activity of encapsulated β-galactosidase compared to a control of free enzyme.
Conclusion
Chitosan/TPP nanoparticles seem to be qualified as a suitable carrier for a targeted delivery of active ingredients to mucosal tissues expressing alkaline phosphatase.
中文翻译:

肠内酶传递:壳聚糖/三聚磷酸盐纳米颗粒可在粘液凝胶屏障后提供靶向释放
目的
这项研究的目的是评估壳聚糖/三聚磷酸(TPP)纳米颗粒在肠粘液凝胶屏障后面提供β-半乳糖苷酶靶向释放的潜力。
方法
在β-半乳糖苷酶的存在下,通过壳聚糖和TPP的离子凝胶化制备了纳米颗粒。表征颗粒的大小,多分散指数和载药量。在与分离的以及与细胞和组织相关的肠碱性磷酸酶(IAP)孵育期间,研究了目标介导的TPP交联剂水解,随后的颗粒降解和β-半乳糖苷酶释放。通过孔雀石测定法对培养基中的磷酸盐含量进行了定量,而通过测量粒径的减小以及在600 nm处的光密度的降低,可以同时监测颗粒的崩解。β-半乳糖苷酶的释放量可通过双辛可宁酸(BCA)蛋白检测或通过以2-硝基苯基β-D-吡喃半乳糖苷(ONPG)为底物的酶活性测定。通过ONPG分析验证了对胰蛋白酶降解的保护作用。
结果
纳米颗粒的大小为573±34 nm,有效载荷为376±18 µgβ-半乳糖苷酶/ mg颗粒。使用分离的IAP进行的降解研究表明,最大的磷酸盐裂解率为118±1 µg / mg颗粒,粒径减小至38±7%,β-半乳糖苷酶释放为58±0.5%。在猪粘膜上孵育3小时后,已证实释放出94±6%的掺入的β-半乳糖苷酶初始量。此外,获得了针对胰蛋白酶降解的保护,导致包封的β-半乳糖苷酶的残留酶活性比游离酶的对照高3倍。
结论
壳聚糖/ TPP纳米颗粒似乎适合作为将活性成分靶向递送至表达碱性磷酸酶的粘膜组织的合适载体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号