当前位置:
X-MOL 学术
›
Eur. J. Pharm. Biopharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modelling the intradermal delivery of microneedle array patches for long-acting antiretrovirals using PBPK.
European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.9 ) Pub Date : 2019-09-13 , DOI: 10.1016/j.ejpb.2019.09.011 Rajith K R Rajoli 1 , Charles Flexner 2 , Justin Chiong 1 , Andrew Owen 1 , Ryan F Donnelly 3 , Eneko Larrañeta 3 , Marco Siccardi 1
European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.9 ) Pub Date : 2019-09-13 , DOI: 10.1016/j.ejpb.2019.09.011 Rajith K R Rajoli 1 , Charles Flexner 2 , Justin Chiong 1 , Andrew Owen 1 , Ryan F Donnelly 3 , Eneko Larrañeta 3 , Marco Siccardi 1
Affiliation
|
INTRODUCTION
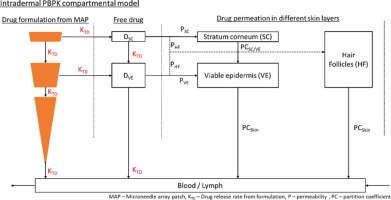
Existing HIV therapy using oral antiretrovirals (ARVs) can result in pill fatigue and sub-optimal adherence. Microneedle array patches (MAPs) offer non-invasive, blood-free and painless drug delivery, and may improve patient adherence. The objective of this study was to develop a novel physiologically-based pharmacokinetic (PBPK) model to simulate the systemic pharmacokinetics of cabotegravir and rilpivirine MAPs using the intradermal route.
METHODS
The developed PBPK models were qualified against observed pharmacokinetic data after intramuscular (IM) and intradermal administration of long-acting nanoformulated rilpivirine to rats, and for IM administration of both drugs to healthy adults. Qualified models were then utilised to estimate suitable MAP characteristics (e.g. nanoformulation dose and release rates) and inform dosing strategies to maintain plasma concentrations above target trough concentrations for the designated dosing interval.
RESULTS
PBPK models simulated q4-weekly loading and maintenance doses of 360 mg and 180 mg for long-acting formulated cabotegravir between the release rates of 1 × 10-3-3 × 10-3h-1 and 1 × 10-3-1.5 × 10-3h-1 respectively, for a 70 kg adult. Estimated patch size was 60 cm2 for a 360 mg dose of cabotegravir. For q4-weekly dosing, rilpivirine required a 1080 mg loading dose and a 540 mg maintenance dose with release rates of 1.5 × 10-3-2.5 × 10-3h-1 and 5 × 10-4-1 × 10-3h-1, respectively. Weekly dosing was also evaluated to assess the potential application from a smaller patch size. The ability to self-administer via a patch that is only left in place for a short duration makes longer durations less important than for some other long-acting approaches. Weekly cabotegravir required 60 mg between release rates 7 × 10-3-9 × 10-3h-1 and rilpivirine required 270 mg and 180 mg respectively between release rates of 7 × 10-3-9 × 10-3h-1.
DISCUSSION
This model estimated optimal dose and release rates for cabotegravir and rilpivirine MAPs. Our approach provides a computational platform to support rational development of intradermal administration strategies to tackle problems associated with chronic oral ARV administration.
中文翻译:

使用 PBPK 模拟用于长效抗逆转录病毒药物的微针阵列贴片的皮内递送。
介绍 使用口服抗逆转录病毒药物 (ARV) 的现有 HIV 治疗会导致药丸疲劳和依从性不佳。微针阵列贴片 (MAP) 提供无创、无血和无痛的药物输送,并可提高患者的依从性。本研究的目的是开发一种新的基于生理学的药代动力学 (PBPK) 模型,以使用皮内途径模拟 cabotegravir 和 rilpivirine MAPs 的全身药代动力学。方法 将开发的 PBPK 模型与在肌肉内 (IM) 和皮内给予大鼠长效纳米制剂利匹韦林后观察到的药代动力学数据以及对健康成人 IM 给予两种药物后的药代动力学数据进行验证。然后使用合格的模型来估计合适的 MAP 特征(例如 纳米制剂剂量和释放率)并告知给药策略,以在指定的给药间隔内将血浆浓度维持在目标谷浓度以上。结果 PBPK 模型模拟了在 1 × 10-3-3 × 10-3h-1 和 1 × 10-3-1.5 × 之间的长效配方 cabotegravir 的第 4 周负荷和维持剂量 360 mg 和 180 mg分别为10-3h-1,为70公斤成人。对于 360 mg 剂量的 cabotegravir,估计贴剂大小为 60 cm2。对于第 4 周给药,利匹韦林需要 1080 mg 负荷剂量和 540 mg 维持剂量,释放速率为 1.5 × 10-3-2.5 × 10-3h-1 和 5 × 10-4-1 × 10-3h-1 , 分别。还评估了每周给药以评估较小贴剂尺寸的潜在应用。通过贴片进行自我管理的能力只保留一小段时间,这使得较长的持续时间不如其他一些长效方法重要。每周 cabotegravir 在 7 × 10-3-9 × 10-3h-1 的释放速率之间需要 60 mg,而利匹韦林在 7 × 10-3-9 × 10-3h-1 的释放速率之间分别需要 270 mg 和 180 mg。讨论 该模型估计了cabotegravir 和rilpivirine MAP 的最佳剂量和释放速率。我们的方法提供了一个计算平台来支持皮内给药策略的合理开发,以解决与慢性口服 ARV 给药相关的问题。每周 cabotegravir 在 7 × 10-3-9 × 10-3h-1 的释放速率之间需要 60 mg,而利匹韦林在 7 × 10-3-9 × 10-3h-1 的释放速率之间分别需要 270 mg 和 180 mg。讨论 该模型估计了cabotegravir 和rilpivirine MAP 的最佳剂量和释放速率。我们的方法提供了一个计算平台来支持皮内给药策略的合理开发,以解决与慢性口服 ARV 给药相关的问题。每周 cabotegravir 在 7 × 10-3-9 × 10-3h-1 的释放速率之间需要 60 mg,而利匹韦林在 7 × 10-3-9 × 10-3h-1 的释放速率之间分别需要 270 mg 和 180 mg。讨论 该模型估计了cabotegravir 和rilpivirine MAP 的最佳剂量和释放速率。我们的方法提供了一个计算平台来支持皮内给药策略的合理开发,以解决与慢性口服 ARV 给药相关的问题。
更新日期:2019-09-13
中文翻译:

使用 PBPK 模拟用于长效抗逆转录病毒药物的微针阵列贴片的皮内递送。
介绍 使用口服抗逆转录病毒药物 (ARV) 的现有 HIV 治疗会导致药丸疲劳和依从性不佳。微针阵列贴片 (MAP) 提供无创、无血和无痛的药物输送,并可提高患者的依从性。本研究的目的是开发一种新的基于生理学的药代动力学 (PBPK) 模型,以使用皮内途径模拟 cabotegravir 和 rilpivirine MAPs 的全身药代动力学。方法 将开发的 PBPK 模型与在肌肉内 (IM) 和皮内给予大鼠长效纳米制剂利匹韦林后观察到的药代动力学数据以及对健康成人 IM 给予两种药物后的药代动力学数据进行验证。然后使用合格的模型来估计合适的 MAP 特征(例如 纳米制剂剂量和释放率)并告知给药策略,以在指定的给药间隔内将血浆浓度维持在目标谷浓度以上。结果 PBPK 模型模拟了在 1 × 10-3-3 × 10-3h-1 和 1 × 10-3-1.5 × 之间的长效配方 cabotegravir 的第 4 周负荷和维持剂量 360 mg 和 180 mg分别为10-3h-1,为70公斤成人。对于 360 mg 剂量的 cabotegravir,估计贴剂大小为 60 cm2。对于第 4 周给药,利匹韦林需要 1080 mg 负荷剂量和 540 mg 维持剂量,释放速率为 1.5 × 10-3-2.5 × 10-3h-1 和 5 × 10-4-1 × 10-3h-1 , 分别。还评估了每周给药以评估较小贴剂尺寸的潜在应用。通过贴片进行自我管理的能力只保留一小段时间,这使得较长的持续时间不如其他一些长效方法重要。每周 cabotegravir 在 7 × 10-3-9 × 10-3h-1 的释放速率之间需要 60 mg,而利匹韦林在 7 × 10-3-9 × 10-3h-1 的释放速率之间分别需要 270 mg 和 180 mg。讨论 该模型估计了cabotegravir 和rilpivirine MAP 的最佳剂量和释放速率。我们的方法提供了一个计算平台来支持皮内给药策略的合理开发,以解决与慢性口服 ARV 给药相关的问题。每周 cabotegravir 在 7 × 10-3-9 × 10-3h-1 的释放速率之间需要 60 mg,而利匹韦林在 7 × 10-3-9 × 10-3h-1 的释放速率之间分别需要 270 mg 和 180 mg。讨论 该模型估计了cabotegravir 和rilpivirine MAP 的最佳剂量和释放速率。我们的方法提供了一个计算平台来支持皮内给药策略的合理开发,以解决与慢性口服 ARV 给药相关的问题。每周 cabotegravir 在 7 × 10-3-9 × 10-3h-1 的释放速率之间需要 60 mg,而利匹韦林在 7 × 10-3-9 × 10-3h-1 的释放速率之间分别需要 270 mg 和 180 mg。讨论 该模型估计了cabotegravir 和rilpivirine MAP 的最佳剂量和释放速率。我们的方法提供了一个计算平台来支持皮内给药策略的合理开发,以解决与慢性口服 ARV 给药相关的问题。



























 京公网安备 11010802027423号
京公网安备 11010802027423号