当前位置:
X-MOL 学术
›
Acta Pharm. Sin. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, in vitro and in vivo biological evaluation of novel lappaconitine derivatives as potential anti-inflammatory agents.
Acta Pharmaceutica Sinica B ( IF 14.5 ) Pub Date : 2019-09-13 , DOI: 10.1016/j.apsb.2019.09.002 Lei Pang 1 , Chun-Yan Liu 2 , Guo-Hua Gong 2 , Zhe-Shan Quan 1
Acta Pharmaceutica Sinica B ( IF 14.5 ) Pub Date : 2019-09-13 , DOI: 10.1016/j.apsb.2019.09.002 Lei Pang 1 , Chun-Yan Liu 2 , Guo-Hua Gong 2 , Zhe-Shan Quan 1
Affiliation
|
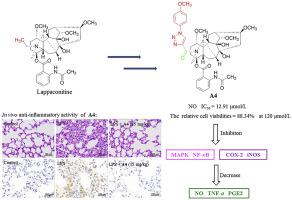
Lappaconitine (LA), a natural compound with a novel C18-diterpenoid alkaloid skeleton, displayed extensive biological profile. Recent research on LA is focused mainly on its anti-tumor and analgesic effects, and therefore we aimed to investigate its anti-inflammatory potential. A series of novel LA derivatives with various substituents on the 20-N position was designed and synthesized. In the initial screening of LA derivatives against NO production, all the target compounds, except compound E2, exhibited excellent inhibitory ability relative to that of LA. Particularly, compound A4 exhibited the most potent inhibition with IC50 of 12.91 μmol/L. The elementary structure-activity relationships (SARs) of NO inhibitory activity indicated that replacement of the benzene ring with an electron donating group could improve the anti-inflammatory efficacy. Furthermore, compound A4 shows an anti-inflammatory mechanism by inhibiting NO, PGE2, and TNF-α generation via the suppression of NF-κB and MAPK signaling pathways. Notably, compound A4 could exert a significant therapeutic effect on LPS-induced acute lung injury (ALI) in vivo. Based on the above research, we further investigated the preliminary pharmacokinetic property of A4 in rats. Therefore, compound A4 could be a promising candidate for the development of anti-inflammatory agents in the future.
中文翻译:

新型拉帕尼汀衍生物作为潜在抗炎药的合成,体外和体内生物学评价。
拉帕尼汀(LA)是一种具有新型C18-二萜生物碱骨架的天然化合物,具有广泛的生物学特性。最近关于LA的研究主要集中在其抗肿瘤和镇痛作用,因此我们旨在研究其抗炎潜力。设计并合成了一系列在20-N位置具有多个取代基的新型LA衍生物。在初步筛选LA衍生物产生NO的过程中,除化合物E2以外,所有目标化合物均具有相对于LA优异的抑制能力。特别是,化合物A4表现出最强的抑制作用,IC50为12.91μmol/ L。NO抑制活性的基本结构-活性关系(SAR)表明,用供电子基团取代苯环可以提高抗炎效果。此外,化合物A4通过抑制NF-κB和MAPK信号通路抑制NO,PGE2和TNF-α的生成,从而显示出抗炎机制。值得注意的是,化合物A4可以在体内对LPS诱导的急性肺损伤(ALI)发挥显着的治疗作用。基于以上研究,我们进一步研究了A4在大鼠中的初步药代动力学特性。因此,化合物A4可能是将来开发抗炎药的有希望的候选物。我们进一步研究了A4在大鼠中的初步药代动力学特性。因此,化合物A4可能是将来开发抗炎药的有希望的候选物。我们进一步研究了A4在大鼠中的初步药代动力学特性。因此,化合物A4可能是将来开发抗炎药的有希望的候选物。
更新日期:2020-04-20
中文翻译:

新型拉帕尼汀衍生物作为潜在抗炎药的合成,体外和体内生物学评价。
拉帕尼汀(LA)是一种具有新型C18-二萜生物碱骨架的天然化合物,具有广泛的生物学特性。最近关于LA的研究主要集中在其抗肿瘤和镇痛作用,因此我们旨在研究其抗炎潜力。设计并合成了一系列在20-N位置具有多个取代基的新型LA衍生物。在初步筛选LA衍生物产生NO的过程中,除化合物E2以外,所有目标化合物均具有相对于LA优异的抑制能力。特别是,化合物A4表现出最强的抑制作用,IC50为12.91μmol/ L。NO抑制活性的基本结构-活性关系(SAR)表明,用供电子基团取代苯环可以提高抗炎效果。此外,化合物A4通过抑制NF-κB和MAPK信号通路抑制NO,PGE2和TNF-α的生成,从而显示出抗炎机制。值得注意的是,化合物A4可以在体内对LPS诱导的急性肺损伤(ALI)发挥显着的治疗作用。基于以上研究,我们进一步研究了A4在大鼠中的初步药代动力学特性。因此,化合物A4可能是将来开发抗炎药的有希望的候选物。我们进一步研究了A4在大鼠中的初步药代动力学特性。因此,化合物A4可能是将来开发抗炎药的有希望的候选物。我们进一步研究了A4在大鼠中的初步药代动力学特性。因此,化合物A4可能是将来开发抗炎药的有希望的候选物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号