当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unique roles of iron and zinc binding to the yeast Fe-S cluster scaffold assembly protein "Isu1".
Metallomics ( IF 3.4 ) Pub Date : 2019-09-09 , DOI: 10.1039/c9mt00172g Brianne E Lewis 1 , Zachary Mason 1 , Andria V Rodrigues 1 , Manunya Nuth 2 , Eric Dizin 2 , J A Cowan 2 , Timothy L Stemmler 1
Metallomics ( IF 3.4 ) Pub Date : 2019-09-09 , DOI: 10.1039/c9mt00172g Brianne E Lewis 1 , Zachary Mason 1 , Andria V Rodrigues 1 , Manunya Nuth 2 , Eric Dizin 2 , J A Cowan 2 , Timothy L Stemmler 1
Affiliation
|
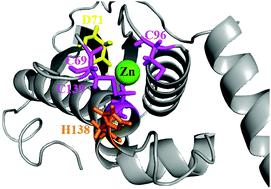
Mitochondrial Fe–S cluster biosynthesis is accomplished within yeast utilizing the biophysical attributes of the “Isu1” scaffold assembly protein. As a member of a highly homologous protein family, Isu1 has sequence conservation between orthologs and a conserved ability to assemble [2Fe–2S] clusters. Regardless of species, scaffold orthologs have been shown to exist in both “disordered” and “structured” conformations, a structural architecture that is directly related to conformations utilized during Fe–S cluster assembly. During assembly, the scaffold helps direct the delivery and utilization of Fe(II) and persulfide substrates to produce [2Fe–2S] clusters, however Zn(II) binding alters the activity of the scaffold while at the same time stabilizes the protein in its structured state. Additional studies confirm Zn binds to the scaffold's Cys rich active site, and has an impact on the protein's ability to make Fe–S clusters. Understanding the interplay between Fe(II) and Zn(II) binding to Isu1 in vitro may help clarify metal loading events that occur during Fe–S cluster assembly in vivo. Here we determine the metal : protein stoichiometry for Isu1 Zn and Fe binding to be 1 : 1 and 2 : 1, respectively. As expected, while Zn binding shifts the Isu1 to its structured state, folding is not influenced by Fe(II) binding. X-ray absorption spectroscopy (XAS) confirms Zn(II) binds to the scaffold's cysteine rich active site but Fe(II) binds at a location distinct from the active site. XAS results show Isu1 binding initially of either Fe(II) or Zn(II) does not significantly perturb the metal site structure of alternate metal. XAS confirmed that four scaffold orthologs bind iron as high-spin Fe(II) at a site composed of ca. 6 oxygen and nitrogen nearest neighbor ligands. Finally, in our report Zn binding dramatically reduces the Fe–S cluster assembly activity of Isu1 even in the presence of frataxin. Given the Fe-binding activity we report for Isu1 and its orthologs here, a possible mechanism involving Fe(II) transport to the scaffold's active site during cluster assembly has been considered.
中文翻译:

铁和锌与酵母Fe-S簇支架组装蛋白“ Isu1”结合的独特作用。
利用“ Isu1”支架组装蛋白的生物物理特性,可以在酵母内完成线粒体Fe–S簇的生物合成。作为高度同源蛋白质家族的成员,Isu1在直系同源基因之间具有序列保守性,并且具有组装[2Fe–2S]簇的保守能力。不论物种如何,支架直向同源物均已显示为“无序”构象和“结构”构象,这是一种与Fe-S团簇组装过程中所使用的构象直接相关的结构体系。在组装过程中,支架有助于指导Fe(II)和过硫化物底物的输送和利用,以产生[2Fe–2S]团簇,而Zn(II)结合改变支架的活性,同时将蛋白质稳定在其结构状态。进一步的研究证实,锌与支架的Cys丰富的活性位点结合,并且对蛋白质形成Fe–S簇的能力有影响。了解Fe(II)和Zn(II)与Isu1结合的相互作用在体外可能有助于弄清在体内Fe–S团簇组装过程中发生的金属负载事件。在这里,我们确定Isu1 Zn和Fe结合的金属:蛋白质化学计量比分别为1:1和2:1。不出所料,虽然Zn结合将Isu1转变为其结构状态,但折叠不受Fe(II)结合的影响。X射线吸收光谱法(XAS)确认Zn(II)与支架的富含半胱氨酸的活性位点结合,但是Fe(II)在与活性位点不同的位置结合。XAS结果表明,Isu1最初与Fe(II)或Zn(II)结合不会显着干扰替代金属的金属位点结构。XAS证实四个支架直系同源物在由ca组成的位点结合铁作为高旋转Fe(II)。6个氧和氮最接近的配体。最后,在我们的报告中,即使在存在frataxin的情况下,Zn结合也会显着降低Isu1的Fe–S簇组装活性。考虑到Fe的结合活性,我们在这里报告Isu1及其直系同源物,这可能是涉及Fe(II)已考虑在集群组装过程中将其运输到支架的活动位点。
更新日期:2019-09-09
中文翻译:

铁和锌与酵母Fe-S簇支架组装蛋白“ Isu1”结合的独特作用。
利用“ Isu1”支架组装蛋白的生物物理特性,可以在酵母内完成线粒体Fe–S簇的生物合成。作为高度同源蛋白质家族的成员,Isu1在直系同源基因之间具有序列保守性,并且具有组装[2Fe–2S]簇的保守能力。不论物种如何,支架直向同源物均已显示为“无序”构象和“结构”构象,这是一种与Fe-S团簇组装过程中所使用的构象直接相关的结构体系。在组装过程中,支架有助于指导Fe(II)和过硫化物底物的输送和利用,以产生[2Fe–2S]团簇,而Zn(II)结合改变支架的活性,同时将蛋白质稳定在其结构状态。进一步的研究证实,锌与支架的Cys丰富的活性位点结合,并且对蛋白质形成Fe–S簇的能力有影响。了解Fe(II)和Zn(II)与Isu1结合的相互作用在体外可能有助于弄清在体内Fe–S团簇组装过程中发生的金属负载事件。在这里,我们确定Isu1 Zn和Fe结合的金属:蛋白质化学计量比分别为1:1和2:1。不出所料,虽然Zn结合将Isu1转变为其结构状态,但折叠不受Fe(II)结合的影响。X射线吸收光谱法(XAS)确认Zn(II)与支架的富含半胱氨酸的活性位点结合,但是Fe(II)在与活性位点不同的位置结合。XAS结果表明,Isu1最初与Fe(II)或Zn(II)结合不会显着干扰替代金属的金属位点结构。XAS证实四个支架直系同源物在由ca组成的位点结合铁作为高旋转Fe(II)。6个氧和氮最接近的配体。最后,在我们的报告中,即使在存在frataxin的情况下,Zn结合也会显着降低Isu1的Fe–S簇组装活性。考虑到Fe的结合活性,我们在这里报告Isu1及其直系同源物,这可能是涉及Fe(II)已考虑在集群组装过程中将其运输到支架的活动位点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号