Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ssy1 functions at the plasma membrane as a receptor of extracellular amino acids independent of plasma membrane-endoplasmic reticulum junctions.
Traffic ( IF 4.5 ) Pub Date : 2019-08-29 , DOI: 10.1111/tra.12681 Andreas Ring 1 , António Martins 1 , Per O Ljungdahl 1
Traffic ( IF 4.5 ) Pub Date : 2019-08-29 , DOI: 10.1111/tra.12681 Andreas Ring 1 , António Martins 1 , Per O Ljungdahl 1
Affiliation
|
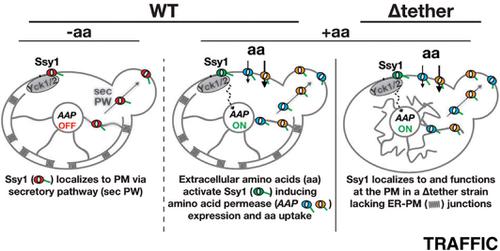
Evidence from multiple laboratories has implicated Ssy1, a nontransporting amino acid permease, as the receptor component of the yeast plasma membrane (PM)-localized SPS (Ssy1-Ptr3-Ssy5)-sensor. Upon binding external amino acids, Ssy1 is thought to initiate signaling events leading to the induction of amino acid permease gene expression. In striking contrast, Kralt et al (2015) (Traffic 16:135-147) have questioned the role of Ssy1 in amino acid sensing and reported that Ssy1 is a component of the endoplasmic reticulum (ER), where it reportedly participates in the formation of ER-PM junctions. Here, we have re-examined the intracellular location of Ssy1 and tested the role of ER-PM junctions in SPS sensor signaling. We show that the C-terminal of Ssy1 carries a functional ER-export motif required for proper localization of Ssy1 to the PM. Furthermore, ER-PM junctions are dispensable for PM-localization and function of Ssy1; Ssy1 localizes to the PM in a Δtether strain lacking ER-PM junctions (ist2Δ scs2Δ scs22Δ tcb1Δ tcb2Δ tcb3Δ), and this strain retains the ability to initiate signals induced by extracellular amino acids. The data demonstrate that Ssy1 functions as the primary amino acid receptor and that it carries out this function at the PM.
中文翻译:

Ssy1在质膜上起细胞外氨基酸受体的作用,独立于质膜-内质网连接。
来自多个实验室的证据表明,Ssy1是一种非运输性氨基酸通透酶,它是酵母质膜(PM)定位的SPS(Ssy1-Ptr3-Ssy5)-传感器的受体成分。结合外部氨基酸后,Ssy1被认为可引发信号传导事件,从而诱导氨基酸渗透酶基因表达。与之形成鲜明对比的是,Kratt等人(2015)(Traffic 16:135-147)质疑Ssy1在氨基酸感测中的作用,并报告说Ssy1是内质网(ER)的成分,据报道Ssy1参与了内质网的形成ER-PM交汇点。在这里,我们已经重新检查了Ssy1的细胞内位置,并测试了ER-PM连接在SPS传感器信号中的作用。我们显示,Ssy1的C末端带有Ssy1正确定位到PM所需的功能性ER出口基序。此外,ER-PM连接对于PM定位和Ssy1的功能是必不可少的。Ssy1在缺少ER-PM连接的Δtether菌株(ist2Δscs2Δscs22Δtcb1Δtcb2Δtcb3Δ)中定位于PM,该菌株保留了启动由细胞外氨基酸诱导的信号的能力。数据表明,Ssy1充当主要氨基酸受体,并且在PM处执行此功能。
更新日期:2019-08-29
中文翻译:

Ssy1在质膜上起细胞外氨基酸受体的作用,独立于质膜-内质网连接。
来自多个实验室的证据表明,Ssy1是一种非运输性氨基酸通透酶,它是酵母质膜(PM)定位的SPS(Ssy1-Ptr3-Ssy5)-传感器的受体成分。结合外部氨基酸后,Ssy1被认为可引发信号传导事件,从而诱导氨基酸渗透酶基因表达。与之形成鲜明对比的是,Kratt等人(2015)(Traffic 16:135-147)质疑Ssy1在氨基酸感测中的作用,并报告说Ssy1是内质网(ER)的成分,据报道Ssy1参与了内质网的形成ER-PM交汇点。在这里,我们已经重新检查了Ssy1的细胞内位置,并测试了ER-PM连接在SPS传感器信号中的作用。我们显示,Ssy1的C末端带有Ssy1正确定位到PM所需的功能性ER出口基序。此外,ER-PM连接对于PM定位和Ssy1的功能是必不可少的。Ssy1在缺少ER-PM连接的Δtether菌株(ist2Δscs2Δscs22Δtcb1Δtcb2Δtcb3Δ)中定位于PM,该菌株保留了启动由细胞外氨基酸诱导的信号的能力。数据表明,Ssy1充当主要氨基酸受体,并且在PM处执行此功能。


























 京公网安备 11010802027423号
京公网安备 11010802027423号