Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A novel computational framework for D(t) from Fluorescence Recovery after Photobleaching data reveals various anomalous diffusion types in live cell membranes.
Traffic ( IF 4.5 ) Pub Date : 2019-10-01 , DOI: 10.1111/tra.12690 Minchul Kang 1 , Charles A Day 2 , Anne K Kenworthy 3, 4, 5
Traffic ( IF 4.5 ) Pub Date : 2019-10-01 , DOI: 10.1111/tra.12690 Minchul Kang 1 , Charles A Day 2 , Anne K Kenworthy 3, 4, 5
Affiliation
|
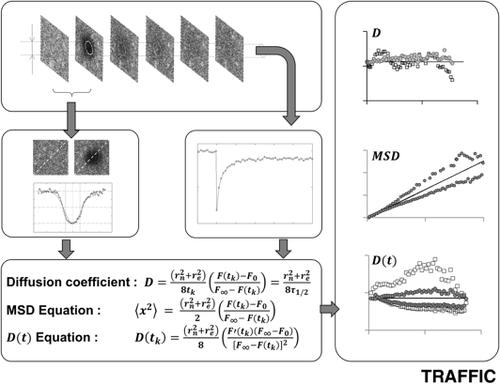
Diffusion of proteins and lipids in lipid membranes plays a pivotal role in almost all aspects of cellular biology, including motility, exo-/endocytosis and signal transduction. For this reason, gaining a detailed understanding of membrane structure and function has long been a major area of cell biology research. To better elucidate this structure-function relationship, various tools have been developed for diffusion measurements, including Fluorescence Recovery After Photobleaching (FRAP). Because of the complexity of cellular microenvironments, biological diffusion is often correlated over time and described by a time-dependent diffusion coefficient, D(t), although the underlying mechanisms are not fully understood. Since D(t) provides important information regarding cellular structures, such as the existence of subresolution barriers to diffusion, many efforts have been made to quantify D(t) by FRAP assuming a single power law, D(t) = Γt α - 1 where Γ and α are transport coefficient and anomalous exponent. However, straightforward approaches to quantify a general form of D(t) are lacking. In this study, we develop a novel mathematical and computational framework to compute the mean square displacement of diffusing molecules and diffusion coefficient D(t) from each individual time point of confocal FRAP data without the single power law assumption. Additionally, we developed an auxiliary equation for D(t) which can readily distinguish normal diffusion or single power law anomalous diffusion from other types of anomalous diffusion directly from FRAP data. Importantly, by applying this approach to FRAP data from a variety of membrane markers, we demonstrate the single power law anomalous diffusion assumption is not sufficient to describe various types of D(t) of membrane proteins. Lastly, we discuss how our new approaches can be applied to other fluorescence microscopy tools such as Fluorescence Correlation Spectroscopy (FCS) and Single Particle Tracking (SPT).
中文翻译:

从漂白数据后的荧光恢复得到的D(t)的新颖计算框架揭示了活细胞膜中的各种异常扩散类型。
蛋白质和脂质在脂质膜中的扩散在细胞生物学的几乎所有方面都起着关键作用,包括运动性,胞外/内吞作用和信号转导。因此,对膜结构和功能的详细了解一直是细胞生物学研究的主要领域。为了更好地阐明这种结构-功能关系,已经开发了用于扩散测量的各种工具,包括光漂白后的荧光恢复(FRAP)。由于细胞微环境的复杂性,生物扩散通常与时间相关,并由时间相关的扩散系数D(t)来描述,尽管尚未完全理解其基本机制。由于D(t)提供有关细胞结构的重要信息,例如存在扩散的亚分辨率障碍,人们已经做出很多努力来通过FRAP量化D(t),假设单个幂定律D(t)=Γtα-1,其中Γ和α是传输系数和异常指数。但是,缺乏量化D(t)的一般形式的直接方法。在这项研究中,我们开发了一种新颖的数学和计算框架,可以在没有单一幂律假设的情况下,从共焦FRAP数据的每个单独时间点计算扩散分子的均方位移和扩散系数D(t)。另外,我们为D(t)开发了一个辅助方程,可以轻松地从FRAP数据中将正态扩散或单幂定律异常扩散与其他类型的异常扩散区分开。重要的,通过将这种方法应用于来自各种膜标记物的FRAP数据,我们证明了单一幂律异常扩散假设不足以描述各种类型的膜蛋白D(t)。最后,我们讨论了如何将我们的新方法应用于其他荧光显微镜工具,例如荧光相关光谱(FCS)和单粒子跟踪(SPT)。
更新日期:2019-10-01
中文翻译:

从漂白数据后的荧光恢复得到的D(t)的新颖计算框架揭示了活细胞膜中的各种异常扩散类型。
蛋白质和脂质在脂质膜中的扩散在细胞生物学的几乎所有方面都起着关键作用,包括运动性,胞外/内吞作用和信号转导。因此,对膜结构和功能的详细了解一直是细胞生物学研究的主要领域。为了更好地阐明这种结构-功能关系,已经开发了用于扩散测量的各种工具,包括光漂白后的荧光恢复(FRAP)。由于细胞微环境的复杂性,生物扩散通常与时间相关,并由时间相关的扩散系数D(t)来描述,尽管尚未完全理解其基本机制。由于D(t)提供有关细胞结构的重要信息,例如存在扩散的亚分辨率障碍,人们已经做出很多努力来通过FRAP量化D(t),假设单个幂定律D(t)=Γtα-1,其中Γ和α是传输系数和异常指数。但是,缺乏量化D(t)的一般形式的直接方法。在这项研究中,我们开发了一种新颖的数学和计算框架,可以在没有单一幂律假设的情况下,从共焦FRAP数据的每个单独时间点计算扩散分子的均方位移和扩散系数D(t)。另外,我们为D(t)开发了一个辅助方程,可以轻松地从FRAP数据中将正态扩散或单幂定律异常扩散与其他类型的异常扩散区分开。重要的,通过将这种方法应用于来自各种膜标记物的FRAP数据,我们证明了单一幂律异常扩散假设不足以描述各种类型的膜蛋白D(t)。最后,我们讨论了如何将我们的新方法应用于其他荧光显微镜工具,例如荧光相关光谱(FCS)和单粒子跟踪(SPT)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号