Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The E. coli HicB Antitoxin Contains a Structurally Stable Helix-Turn-Helix DNA Binding Domain.
Structure ( IF 5.7 ) Pub Date : 2019-09-05 , DOI: 10.1016/j.str.2019.08.008 Melek Cemre Manav 1 , Kathryn Jane Turnbull 2 , Dukas Jurėnas 3 , Abel Garcia-Pino 4 , Kenn Gerdes 2 , Ditlev Egeskov Brodersen 1
Structure ( IF 5.7 ) Pub Date : 2019-09-05 , DOI: 10.1016/j.str.2019.08.008 Melek Cemre Manav 1 , Kathryn Jane Turnbull 2 , Dukas Jurėnas 3 , Abel Garcia-Pino 4 , Kenn Gerdes 2 , Ditlev Egeskov Brodersen 1
Affiliation
|
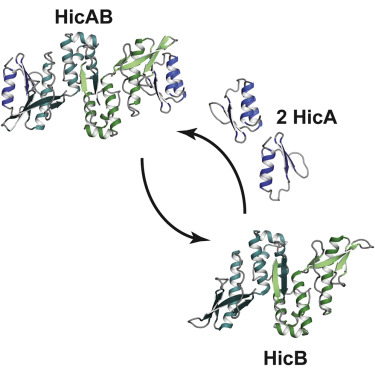
The E. coli hicAB type II toxin-antitoxin locus is unusual by being controlled by two promoters and by having the toxin encoded upstream of the antitoxin. HicA toxins contain a double-stranded RNA-binding fold and cleaves both mRNA and tmRNA in vivo, while HicB antitoxins contain a partial RNase H fold and either a helix-turn-helix (HTH) or ribbon-helix-helix domain. It is not known how an HTH DNA-binding domain affects higher-order structure for the HicAB modules. Here, we present crystal structures of the isolated E. coli HicB antitoxin and full-length HicAB complex showing that HicB forms a stable DNA-binding module and interacts in a canonical way with HicA despite the presence of an HTH-type DNA-binding domain. No major structural rearrangements take place upon binding of the toxin. Both structures expose well-ordered DNA-binding motifs allowing a model for DNA binding by the antitoxin to be generated.
中文翻译:

大肠杆菌HicB抗毒素包含结构稳定的Helix-Turn-Helix DNA结合结构域。
大肠杆菌hicAB II型毒素-抗毒素基因座是不寻常的,受两个启动子控制,并且毒素被编码在抗毒素的上游。HicA毒素包含双链RNA结合折叠并在体内切割mRNA和tmRNA,而HicB抗毒素则包含部分RNase H折叠和螺旋-转-螺旋(HTH)或带状-螺旋-螺旋结构域。尚不知道HTH DNA结合结构域如何影响HicAB模块的高级结构。在这里,我们介绍了分离的大肠杆菌HicB抗毒素和全长HicAB复合物的晶体结构,表明尽管存在HTH型DNA结合域,HicB仍能形成稳定的DNA结合模块并以正常方式与HicA相互作用。毒素结合后不会发生重大的结构重排。
更新日期:2019-09-05
中文翻译:

大肠杆菌HicB抗毒素包含结构稳定的Helix-Turn-Helix DNA结合结构域。
大肠杆菌hicAB II型毒素-抗毒素基因座是不寻常的,受两个启动子控制,并且毒素被编码在抗毒素的上游。HicA毒素包含双链RNA结合折叠并在体内切割mRNA和tmRNA,而HicB抗毒素则包含部分RNase H折叠和螺旋-转-螺旋(HTH)或带状-螺旋-螺旋结构域。尚不知道HTH DNA结合结构域如何影响HicAB模块的高级结构。在这里,我们介绍了分离的大肠杆菌HicB抗毒素和全长HicAB复合物的晶体结构,表明尽管存在HTH型DNA结合域,HicB仍能形成稳定的DNA结合模块并以正常方式与HicA相互作用。毒素结合后不会发生重大的结构重排。


























 京公网安备 11010802027423号
京公网安备 11010802027423号