Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial
The Lancet ( IF 168.9 ) Pub Date : 2019-08-08 , DOI: 10.1016/s0140-6736(19)31773-8 Kristian Reich , April W Armstrong , Richard G Langley , Susan Flavin , Bruce Randazzo , Shu Li , Ming-Chun Hsu , Patrick Branigan , Andrew Blauvelt
中文翻译:

Guselkumab与secukinumab联合治疗中度至重度牛皮癣(ECLIPSE):3期随机对照试验的结果
更新日期:2019-09-06
The Lancet ( IF 168.9 ) Pub Date : 2019-08-08 , DOI: 10.1016/s0140-6736(19)31773-8 Kristian Reich , April W Armstrong , Richard G Langley , Susan Flavin , Bruce Randazzo , Shu Li , Ming-Chun Hsu , Patrick Branigan , Andrew Blauvelt
|
Background
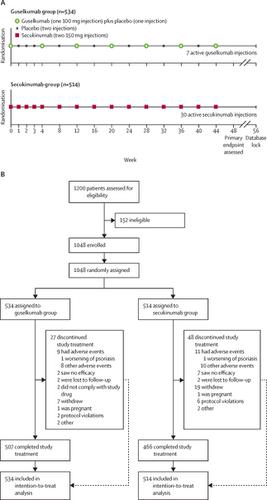
Antibodies targeting interleukin (IL)-23 and IL-17A effectively treat moderate-to-severe psoriasis. ECLIPSE is the first comparator study of an IL-23p19 inhibitor, guselkumab, versus an IL-17A inhibitor, secukinumab. The primary objective of this study was to show superiority of clinical response at week 48 for guselkumab versus secukinumab.Methods
In this phase 3, multicentre, double-blind, randomised, comparator-controlled trial at 142 outpatient clinical sites in nine countries (Australia, Canada, Czech Republic, France, Germany, Hungary, Poland, Spain, and the USA), eligible patients were aged 18 years or older, had moderate-to-severe plaque-type psoriasis, and were candidates for phototherapy or systemic therapy. Eligible patients were randomly assigned with permuted block randomisation using an interactive web response system to receive either guselkumab (100 mg at weeks 0 and 4 then every 8 weeks) or secukinumab (300 mg at weeks 0, 1, 2, 3, and 4, and then every 4 weeks). The primary endpoint, the proportion of patients in the intention-to-treat population who achieved 90% reduction or more from baseline of Psoriasis Area and Severity Index (PASI 90 response) at week 48, and major secondary endpoints (the proportions of patients in the guselkumab group and in the secukinumab group who achieved a PASI 75 response at both weeks 12 and 48, a PASI 90 response at week 12, a PASI 75 response at week 12, a PASI 100 response at week 48, an Investigator's Global Assessment [IGA] score of 0 [cleared] at week 48, and an IGA score of 0 or 1 [minimal] at week 48) were to be tested in a fixed sequence to control type I error rate. Safety was evaluated in patients who received one or more doses of study drug from week 0 to 56. The study is registered with , .Findings
This study was done between April 27, 2017, and Sept 20, 2018. 1048 eligible patients were enrolled and, of these, 534 were assigned to receive guselkumab and 514 to receive secukinumab. The proportion of patients with a PASI 90 response at week 48 was greater in the guselkumab group (451 [84%]) than in the secukinumab group (360 [70%]; p<0·0001). Although non-inferiority (margin of 10 percentage points) was established for the first major secondary endpoint (452 [85%] of patients in the guselkumab group vs 412 [80%] of patients in the secukinumab group achieving a PASI 75 response at both weeks 12 and 48), superiority was not established (p=0·0616). Consequently, formal statistical testing was not done for subsequent major secondary endpoints. Proportions of patients with adverse events, infections, and serious adverse events were similar between the two treatments and, in general, safety findings were consistent with registrational trial observations.Interpretation
Guselkumab showed superior long-term efficacy based on PASI 90 at week 48 when compared with secukinumab for treating moderate-to-severe psoriasis. This finding could assist health-care providers in their decision making process when selecting a biologic for treating moderate-to-severe psoriasis.Funding
This study was funded by Janssen Research & Development.中文翻译:

Guselkumab与secukinumab联合治疗中度至重度牛皮癣(ECLIPSE):3期随机对照试验的结果


























 京公网安备 11010802027423号
京公网安备 11010802027423号