Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The case for class II bacteriocins: A biophysical approach using "suicide probes" in receptor-free hosts to study their mechanism of action.
Biochimie ( IF 3.9 ) Pub Date : 2019-08-02 , DOI: 10.1016/j.biochi.2019.07.024 N S Ríos Colombo 1 , M C Chalón 1 , F G Dupuy 1 , C F Gonzalez 2 , A Bellomio 1
Biochimie ( IF 3.9 ) Pub Date : 2019-08-02 , DOI: 10.1016/j.biochi.2019.07.024 N S Ríos Colombo 1 , M C Chalón 1 , F G Dupuy 1 , C F Gonzalez 2 , A Bellomio 1
Affiliation
|
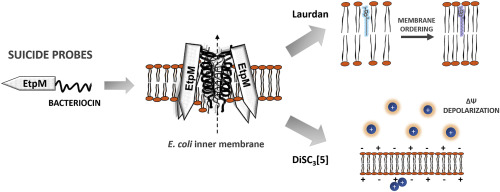
Class II bacteriocins are unmodified membrane-active peptides that act over a narrow spectrum of target bacteria. They bind a specific receptor protein on the membrane to form a pore, leading to membrane permeabilization and cell death. However, little is known about the molecular events triggering the pore formation after the bacteriocin recognizes the receptor. It is not clear yet if the pore is the same receptor forced into an open conformation or if the pore results from the bacteriocin insertion and oligomeric assembly in the lipid bilayer. In order to reveal which model is more suitable to explain the toxicity mechanism, in this work we use chimeric peptides, resulting from the fusion of the bitopic membrane protein EtpM with different class II bacteriocins: enterocin CRL35, pediocin PA-1 and microcin V. E. coli strains lacking the specific receptors for these bacteriocins were chosen as expression hosts. As these constructs display a lethal effect when they are heterologously expressed, they are called "suicide probes". The results suggest that, indeed, the specific receptor would act as a docking molecule more than as a structural piece of the pore, as long as the bacteriocin is somehow anchored to the membrane. These set of chimeric peptides also represent an in vivo system that allows to study the interaction of the bacteriocins with real bacterial membranes, instead of model membranes. Hence, the effects of these suicide probes in membrane fluidity and transmembrane potential were also assessed, using fluorescence spectroscopy. The data show that the different suicide probes are able to increase phospholipid order and depolarize the membranes of receptor-free bacterial cells.
中文翻译:

II类细菌素的案例:一种在无受体宿主中使用“自杀探针”的生物物理方法,以研究其作用机理。
II类细菌素是未修饰的膜活性肽,可作用于狭窄范围的靶细菌。它们与膜上的特定受体蛋白结合形成孔,导致膜透化和细胞死亡。然而,关于细菌素识别受体后触发孔形成的分子事件知之甚少。尚不清楚孔是否是被迫进入开放构象的同一受体,还是孔是由于脂质双层中细菌素的插入和寡聚体组装而产生的。为了揭示哪种模型更适合解释毒性机制,在这项工作中,我们使用了嵌合肽,这些肽是由双位膜蛋白EtpM与不同的II类细菌素:肠球菌素CRL35,pediocin PA-1和microcin VE融合而成的 选择缺乏这些细菌素特异性受体的大肠杆菌菌株作为表达宿主。由于这些构建体在异源表达时显示出致死作用,因此被称为“自杀探针”。结果表明,实际上,只要细菌素以某种方式锚定在膜上,特异性受体将更起着对接分子的作用,而不是充当孔的结构部分。这些嵌合肽组还代表一种体内系统,该系统允许研究细菌素与真实细菌膜而非模型膜的相互作用。因此,还使用荧光光谱法评估了这些自杀探针对膜流动性和跨膜电位的影响。
更新日期:2019-08-02
中文翻译:

II类细菌素的案例:一种在无受体宿主中使用“自杀探针”的生物物理方法,以研究其作用机理。
II类细菌素是未修饰的膜活性肽,可作用于狭窄范围的靶细菌。它们与膜上的特定受体蛋白结合形成孔,导致膜透化和细胞死亡。然而,关于细菌素识别受体后触发孔形成的分子事件知之甚少。尚不清楚孔是否是被迫进入开放构象的同一受体,还是孔是由于脂质双层中细菌素的插入和寡聚体组装而产生的。为了揭示哪种模型更适合解释毒性机制,在这项工作中,我们使用了嵌合肽,这些肽是由双位膜蛋白EtpM与不同的II类细菌素:肠球菌素CRL35,pediocin PA-1和microcin VE融合而成的 选择缺乏这些细菌素特异性受体的大肠杆菌菌株作为表达宿主。由于这些构建体在异源表达时显示出致死作用,因此被称为“自杀探针”。结果表明,实际上,只要细菌素以某种方式锚定在膜上,特异性受体将更起着对接分子的作用,而不是充当孔的结构部分。这些嵌合肽组还代表一种体内系统,该系统允许研究细菌素与真实细菌膜而非模型膜的相互作用。因此,还使用荧光光谱法评估了这些自杀探针对膜流动性和跨膜电位的影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号