当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mutant-Specific Targeting of Ras G12C Activity by Covalently Reacting Small Molecules.
Cell Chemical Biology ( IF 8.6 ) Pub Date : 2019-08-01 , DOI: 10.1016/j.chembiol.2019.07.005 Roger S Goody 1 , Matthias P Müller 2 , Daniel Rauh 2
Cell Chemical Biology ( IF 8.6 ) Pub Date : 2019-08-01 , DOI: 10.1016/j.chembiol.2019.07.005 Roger S Goody 1 , Matthias P Müller 2 , Daniel Rauh 2
Affiliation
|
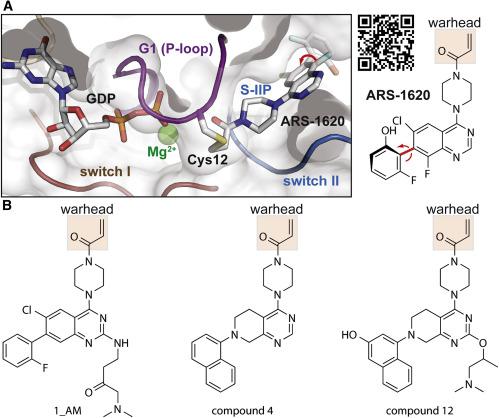
In this review we discuss and compare recently introduced molecules that are able to react covalently with an oncogenic mutant of KRas, KRas G12C. Two different classes of compounds in question have been developed, both leading to the mutant being locked in the inactive (guanosine diphosphate [GDP]-bound) state. The first are compounds that interact reversibly with the switch-II pocket (S-IIP) before covalent interaction. The second class interact in a competitive manner with the GDP/guanosine triphosphate (GTP) binding site. The fundamental physico-chemical principles of the two inhibitor classes are evaluated. For GDP/GTP-competing molecules, we show that special attention must be paid to the influence of guanine nucleotide exchange factors (GEFs) and their elevated activity in cells harboring abnormally activated Ras mutants. A new approach is suggested involving compounds that interact with the guanine binding site of the GTPase, but in a manner that is independent of the interaction of the GTPase with its cognate GEF.
中文翻译:

通过共价反应小分子的Ras G12C活性的特定于突变体的目标。
在这篇综述中,我们讨论并比较了最近引入的能够与KRas致癌突变体KRas G12C共价反应的分子。已经开发出两类不同的化合物,两者都导致突变体被锁定在非活性状态(鸟苷二磷酸[GDP]结合)。第一种是在共价相互作用前与switch-II口袋(S-IIP)可逆相互作用的化合物。第二类以竞争方式与GDP /三磷酸鸟苷(GTP)结合位点相互作用。对这两种抑制剂的基本理化原理进行了评估。对于GDP / GTP竞争分子,我们表明必须特别注意鸟嘌呤核苷酸交换因子(GEFs)的影响及其在带有异常激活的Ras突变体的细胞中的活性升高。
更新日期:2019-11-09
中文翻译:

通过共价反应小分子的Ras G12C活性的特定于突变体的目标。
在这篇综述中,我们讨论并比较了最近引入的能够与KRas致癌突变体KRas G12C共价反应的分子。已经开发出两类不同的化合物,两者都导致突变体被锁定在非活性状态(鸟苷二磷酸[GDP]结合)。第一种是在共价相互作用前与switch-II口袋(S-IIP)可逆相互作用的化合物。第二类以竞争方式与GDP /三磷酸鸟苷(GTP)结合位点相互作用。对这两种抑制剂的基本理化原理进行了评估。对于GDP / GTP竞争分子,我们表明必须特别注意鸟嘌呤核苷酸交换因子(GEFs)的影响及其在带有异常激活的Ras突变体的细胞中的活性升高。



























 京公网安备 11010802027423号
京公网安备 11010802027423号