当前位置:
X-MOL 学术
›
Acta Pharmacol. Sin.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Intravenous formulation of Panax notoginseng root extract: human pharmacokinetics of ginsenosides and potential for perpetrating drug interactions.
Acta Pharmacologica Sinica ( IF 8.2 ) Pub Date : 2019-07-29 , DOI: 10.1038/s41401-019-0273-1 Salisa Pintusophon 1, 2 , Wei Niu 1 , Xiao-Na Duan 1, 2 , Olajide E Olaleye 1 , Yu-Hong Huang 3 , Feng-Qing Wang 1 , Yan-Fen Li 3 , Jun-Ling Yang 1 , Chuan Li 1, 2
Acta Pharmacologica Sinica ( IF 8.2 ) Pub Date : 2019-07-29 , DOI: 10.1038/s41401-019-0273-1 Salisa Pintusophon 1, 2 , Wei Niu 1 , Xiao-Na Duan 1, 2 , Olajide E Olaleye 1 , Yu-Hong Huang 3 , Feng-Qing Wang 1 , Yan-Fen Li 3 , Jun-Ling Yang 1 , Chuan Li 1, 2
Affiliation
|
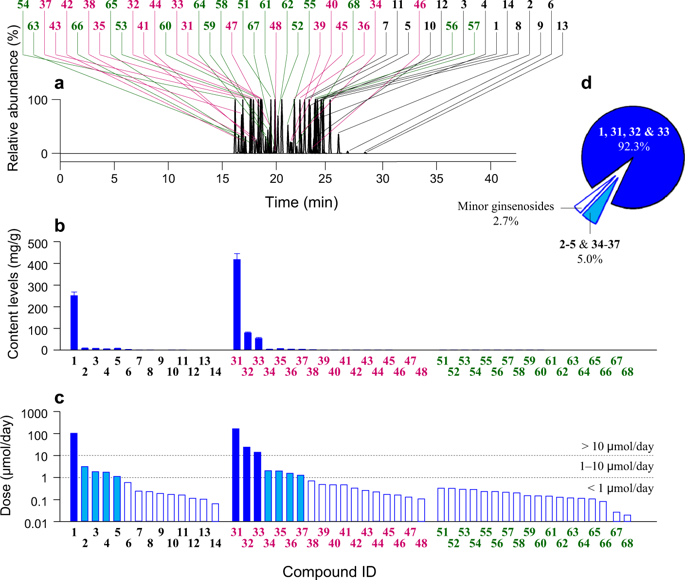
XueShuanTong, a lyophilized extract of Panax notoginseng roots (Sanqi) for intravenous administration, is extensively used as add-on therapy in the treatment of ischemic heart and cerebrovascular diseases and comprises therapeutically active ginsenosides. Potential for XueShuanTong-drug interactions was determined; the investigation focused on cytochrome P450 (CYP)3A induction and organic anion-transporting polypeptide (OATP)1B inhibition. Ginsenosides considerably bioavailable for drug interactions were identified by dosing XueShuanTong in human subjects and their interaction-related pharmacokinetics were determined. The CYP3A induction potential was determined by repeatedly dosing XueShuanTong for 15 days in human subjects and by treating cryopreserved human hepatocytes with circulating ginsenosides; midazolam served as a probe substrate. Joint inhibition of OATP1B by XueShuanTong ginsenosides was assessed in vitro, and the data were processed using the Chou-Talalay method. Samples were analyzed by liquid chromatography/mass spectrometry. Ginsenosides Rb1, Rd, and Rg1 and notoginsenoside R1 were the major circulating XueShuanTong compounds; their interaction-related pharmacokinetics comprised compound dose-dependent levels of systemic exposure and, for ginsenosides Rb1 and Rd, long terminal half-lives (32‒57 and 58‒307 h, respectively) and low unbound fractions in plasma (0.8%‒2.9% and 0.4%‒3.0%, respectively). Dosing XueShuanTong did not induce CYP3A. Based on the pharmacokinetics and inhibitory potency of the ginsenosides, XueShuanTong was predicted to have high potential for OATP1B3-mediated drug interactions (attributed chiefly to ginsenoside Rb1) suggesting the need for further model-based determination of the interaction potential for XueShuanTong and, if necessary, a clinical drug interaction study. Increased awareness of ginsenosides' pharmacokinetics and XueShuanTong-drug interaction potential will help ensure the safe use of XueShuanTong and coadministered synthetic drugs.
中文翻译:

三七根提取物的静脉制剂:人参皂甙的人药代动力学和进行药物相互作用的潜力。
血栓通是一种三七人参的冻干提取物,用于静脉内给药,已广泛用作治疗缺血性心脏病和脑血管疾病的附加疗法,并且包含具有治疗活性的人参皂甙。确定了血栓通-药物相互作用的潜力;该研究集中于细胞色素P450(CYP)3A的诱导和有机阴离子转运多肽(OATP)1B的抑制。通过在人受试者中服用血栓通剂鉴定了可用于药物相互作用的可生物利用度极高的人参皂苷,并测定了它们与相互作用有关的药代动力学。CYP3A的诱导潜力是通过在人类受试者中反复给药血栓通15天并用循环的人参皂苷处理冷冻保存的人类肝细胞来确定的。咪达唑仑用作探针底物。体外评估血栓通人参皂甙对OATP1B的联合抑制作用,并使用Chou-Talalay方法处理数据。通过液相色谱/质谱法分析样品。人参皂苷Rb1,Rd,Rg1和三七皂苷R1是主要的循环血栓通化合物。它们的相互作用相关的药代动力学包括剂量依赖性的全身暴露水平,对于人参皂苷Rb1和Rd,其半衰期长(分别为32‒57和58‒307 h)和血浆中的未结合部分较低(0.8%‒2.9) %和0.4%and3.0%)。给药血栓通未诱导CYP3A。根据人参皂苷的药代动力学和抑制力,据预测,血栓通在OATP1B3介导的药物相互作用中具有很高的潜力(主要归因于人参皂甙Rb1),这表明需要进一步基于模型确定血栓通的相互作用潜能,并在必要时进行临床药物相互作用研究。人参皂苷的药代动力学和血栓通-药物相互作用潜能的增强认识将有助于确保安全使用血栓通和合成药物。
更新日期:2019-11-18
中文翻译:

三七根提取物的静脉制剂:人参皂甙的人药代动力学和进行药物相互作用的潜力。
血栓通是一种三七人参的冻干提取物,用于静脉内给药,已广泛用作治疗缺血性心脏病和脑血管疾病的附加疗法,并且包含具有治疗活性的人参皂甙。确定了血栓通-药物相互作用的潜力;该研究集中于细胞色素P450(CYP)3A的诱导和有机阴离子转运多肽(OATP)1B的抑制。通过在人受试者中服用血栓通剂鉴定了可用于药物相互作用的可生物利用度极高的人参皂苷,并测定了它们与相互作用有关的药代动力学。CYP3A的诱导潜力是通过在人类受试者中反复给药血栓通15天并用循环的人参皂苷处理冷冻保存的人类肝细胞来确定的。咪达唑仑用作探针底物。体外评估血栓通人参皂甙对OATP1B的联合抑制作用,并使用Chou-Talalay方法处理数据。通过液相色谱/质谱法分析样品。人参皂苷Rb1,Rd,Rg1和三七皂苷R1是主要的循环血栓通化合物。它们的相互作用相关的药代动力学包括剂量依赖性的全身暴露水平,对于人参皂苷Rb1和Rd,其半衰期长(分别为32‒57和58‒307 h)和血浆中的未结合部分较低(0.8%‒2.9) %和0.4%and3.0%)。给药血栓通未诱导CYP3A。根据人参皂苷的药代动力学和抑制力,据预测,血栓通在OATP1B3介导的药物相互作用中具有很高的潜力(主要归因于人参皂甙Rb1),这表明需要进一步基于模型确定血栓通的相互作用潜能,并在必要时进行临床药物相互作用研究。人参皂苷的药代动力学和血栓通-药物相互作用潜能的增强认识将有助于确保安全使用血栓通和合成药物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号