npj Digital Medicine ( IF 15.2 ) Pub Date : 2019-07-26 , DOI: 10.1038/s41746-019-0148-3 Pratik Shah , Francis Kendall , Sean Khozin , Ryan Goosen , Jianying Hu , Jason Laramie , Michael Ringel , Nicholas Schork
|
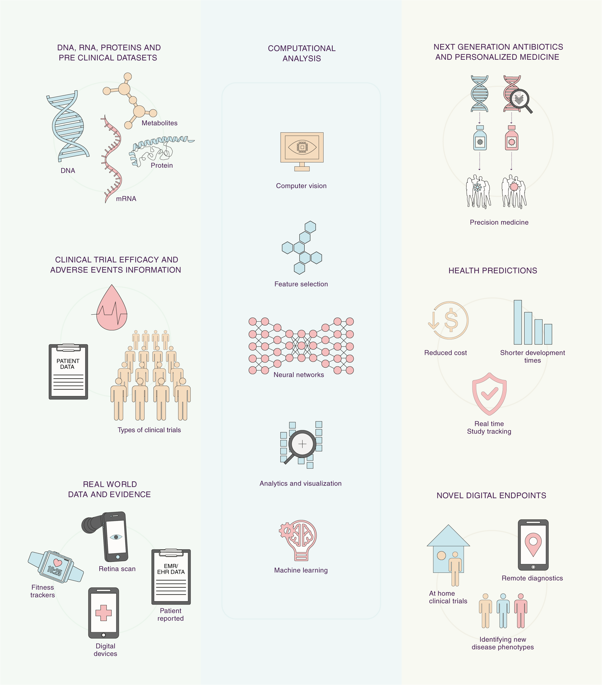
Future of clinical development is on the verge of a major transformation due to convergence of large new digital data sources, computing power to identify clinically meaningful patterns in the data using efficient artificial intelligence and machine-learning algorithms, and regulators embracing this change through new collaborations. This perspective summarizes insights, recent developments, and recommendations for infusing actionable computational evidence into clinical development and health care from academy, biotechnology industry, nonprofit foundations, regulators, and technology corporations. Analysis and learning from publically available biomedical and clinical trial data sets, real-world evidence from sensors, and health records by machine-learning architectures are discussed. Strategies for modernizing the clinical development process by integration of AI- and ML-based digital methods and secure computing technologies through recently announced regulatory pathways at the United States Food and Drug Administration are outlined. We conclude by discussing applications and impact of digital algorithmic evidence to improve medical care for patients.
中文翻译:

人工智能和机器学习在临床开发中的转化视角
由于大型新的数字数据源的融合,强大的计算能力,使用高效的人工智能和机器学习算法来识别数据中具有临床意义的模式的计算能力以及监管机构通过新的合作来拥抱这种变化,临床发展的未来已接近重大变革的边缘。该观点总结了来自学术界,生物技术行业,非营利基金会,监管机构和技术公司的见解,最新进展以及为将可行的计算证据注入临床开发和医疗保健的建议。讨论了从公开可用的生物医学和临床试验数据集进行分析和学习,来自传感器的真实世界证据以及通过机器学习架构的健康记录。概述了通过最近在美国食品和药物管理局宣布的监管途径,通过整合基于AI和ML的数字方法和安全计算技术来实现临床开发过程现代化的策略。最后,我们讨论了数字算法证据在改善患者医疗保健方面的应用和影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号