当前位置:
X-MOL 学术
›
Acta Pharmacol. Sin.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crosstalk between the Akt/mTORC1 and NF-κB signaling pathways promotes hypoxia-induced pulmonary hypertension by increasing DPP4 expression in PASMCs.
Acta Pharmacologica Sinica ( IF 8.2 ) Pub Date : 2019-07-17 , DOI: 10.1038/s41401-019-0272-2 Ying Li 1, 2 , Li Yang 3 , Liang Dong 1 , Zhi-Wei Yang 4 , Jing Zhang 1 , Sheng-Li Zhang 4 , Meng-Jie Niu 5 , Jing-Wen Xia 1 , Yi Gong 1 , Ning Zhu 1 , Xiu-Juan Zhang 1 , Yuan-Yuan Zhang 1 , Xiao-Min Wei 1 , You-Zhi Zhang 1 , Peng Zhang 1 , Sheng-Qing Li 1
Acta Pharmacologica Sinica ( IF 8.2 ) Pub Date : 2019-07-17 , DOI: 10.1038/s41401-019-0272-2 Ying Li 1, 2 , Li Yang 3 , Liang Dong 1 , Zhi-Wei Yang 4 , Jing Zhang 1 , Sheng-Li Zhang 4 , Meng-Jie Niu 5 , Jing-Wen Xia 1 , Yi Gong 1 , Ning Zhu 1 , Xiu-Juan Zhang 1 , Yuan-Yuan Zhang 1 , Xiao-Min Wei 1 , You-Zhi Zhang 1 , Peng Zhang 1 , Sheng-Qing Li 1
Affiliation
|
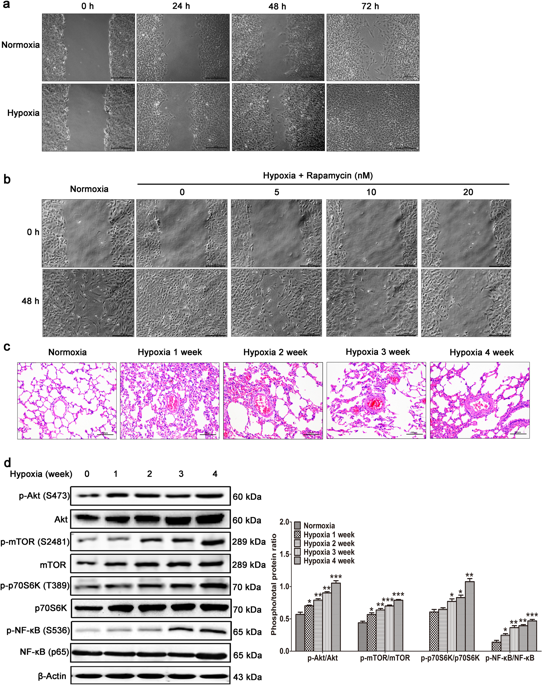
Abnormal wound healing by pulmonary artery smooth muscle cells (PASMCs) promotes vascular remodeling in hypoxia-induced pulmonary hypertension (HPH). Increasing evidence shows that both the mammalian target of rapamycin complex 1 (mTORC1) and nuclear factor-kappa B (NF-κB) are involved in the development of HPH. In this study, we explored the crosstalk between mTORC1 and NF-κB in PASMCs cultured under hypoxic condition and in a rat model of hypoxia-induced pulmonary hypertension (HPH). We showed that hypoxia promoted wound healing of PASMCs, which was dose-dependently blocked by the mTORC1 inhibitor rapamycin (5-20 nM). In PASMCs, hypoxia activated mTORC1, which in turn promoted the phosphorylation of NF-κB. Molecular docking revealed that mTOR interacted with IκB kinases (IKKs) and that was validated by immunoprecipitation. In vitro kinase assays and mass spectrometry demonstrated that mTOR phosphorylated IKKα and IKKβ separately. Inhibition of mTORC1 decreased the level of phosphorylated IKKα/β, thus reducing the phosphorylation and transcriptional activity of NF-κB. Bioinformatics study revealed that dipeptidyl peptidase-4 (DPP4) was a target gene of NF-κB; DPP4 inhibitor, sitagliptin (10-500 μM) effectively inhibited the abnormal wound healing of PASMCs under hypoxic condition. In the rat model of HPH, we showed that NF-κB activation (at 3 weeks) was preceded by mTOR signaling activation (after 1 or 2 weeks) in lungs, and administration of sitagliptin (1-5 mg/kg every day, ig) produced preventive effects against the development of HPH. In conclusion, hypoxia activates the crosstalk between mTORC1 and NF-κB, and increased DPP4 expression in PASMCs that leads to vascular remodeling. Sitagliptin, a DPP4 inhibitor, exerts preventive effect against HPH.
中文翻译:

Akt / mTORC1和NF-κB信号通路之间的串扰通过增加PASMCs中DPP4的表达促进缺氧诱导的肺动脉高压。
肺动脉平滑肌细胞(PASMC)异常的伤口愈合促进了缺氧诱导的肺动脉高压(HPH)中的血管重塑。越来越多的证据表明,雷帕霉素复合物1(mTORC1)和核因子-κB(NF-κB)的哺乳动物靶标都参与了HPH的发展。在这项研究中,我们探讨了低氧条件下培养的PASMCs和低氧诱导的肺动脉高压(HPH)大鼠模型中mTORC1与NF-κB之间的串扰。我们发现缺氧促进了PASMCs的伤口愈合,这被mTORC1抑制剂雷帕霉素(5-20 nM)剂量依赖性地阻断。在PASMC中,缺氧激活mTORC1,进而促进NF-κB的磷酸化。分子对接揭示了mTOR与IκB激酶(IKKs)相互作用,并通过免疫沉淀进行了验证。体外激酶测定和质谱表明,mTOR分别磷酸化了IKKα和IKKβ。抑制mTORC1降低了磷酸化IKKα/β的水平,从而降低了NF-κB的磷酸化和转录活性。生物信息学研究表明,二肽基肽酶-4(DPP4)是NF-κB的靶基因。DPP4抑制剂西他列汀(10-500μM)在缺氧条件下可有效抑制PASMCs的异常伤口愈合。在HPH大鼠模型中,我们显示NF-κB激活(在3周时)先于肺中mTOR信号激活(在1或2周后),然后给予西他列汀(每天1-5 mg / kg,ig )对HPH的产生产生了预防作用。总之,缺氧激活了mTORC1与NF-κB之间的串扰,并导致PASMC中DPP4表达增加,从而导致血管重塑。西他列汀,一种DPP4抑制剂,对HPH具有预防作用。
更新日期:2019-11-18
中文翻译:

Akt / mTORC1和NF-κB信号通路之间的串扰通过增加PASMCs中DPP4的表达促进缺氧诱导的肺动脉高压。
肺动脉平滑肌细胞(PASMC)异常的伤口愈合促进了缺氧诱导的肺动脉高压(HPH)中的血管重塑。越来越多的证据表明,雷帕霉素复合物1(mTORC1)和核因子-κB(NF-κB)的哺乳动物靶标都参与了HPH的发展。在这项研究中,我们探讨了低氧条件下培养的PASMCs和低氧诱导的肺动脉高压(HPH)大鼠模型中mTORC1与NF-κB之间的串扰。我们发现缺氧促进了PASMCs的伤口愈合,这被mTORC1抑制剂雷帕霉素(5-20 nM)剂量依赖性地阻断。在PASMC中,缺氧激活mTORC1,进而促进NF-κB的磷酸化。分子对接揭示了mTOR与IκB激酶(IKKs)相互作用,并通过免疫沉淀进行了验证。体外激酶测定和质谱表明,mTOR分别磷酸化了IKKα和IKKβ。抑制mTORC1降低了磷酸化IKKα/β的水平,从而降低了NF-κB的磷酸化和转录活性。生物信息学研究表明,二肽基肽酶-4(DPP4)是NF-κB的靶基因。DPP4抑制剂西他列汀(10-500μM)在缺氧条件下可有效抑制PASMCs的异常伤口愈合。在HPH大鼠模型中,我们显示NF-κB激活(在3周时)先于肺中mTOR信号激活(在1或2周后),然后给予西他列汀(每天1-5 mg / kg,ig )对HPH的产生产生了预防作用。总之,缺氧激活了mTORC1与NF-κB之间的串扰,并导致PASMC中DPP4表达增加,从而导致血管重塑。西他列汀,一种DPP4抑制剂,对HPH具有预防作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号