Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Arginine-stimulated copeptin measurements in the differential diagnosis of diabetes insipidus: a prospective diagnostic study.
The Lancet ( IF 168.9 ) Pub Date : 2019-07-11 , DOI: 10.1016/s0140-6736(19)31255-3 Bettina Winzeler 1 , Nicole Cesana-Nigro 1 , Julie Refardt 1 , Deborah R Vogt 2 , Cornelia Imber 1 , Benedict Morin 1 , Milica Popovic 1 , Michelle Steinmetz 1 , Clara O Sailer 1 , Gabor Szinnai 3 , Irina Chifu 4 , Martin Fassnacht 5 , Mirjam Christ-Crain 1
The Lancet ( IF 168.9 ) Pub Date : 2019-07-11 , DOI: 10.1016/s0140-6736(19)31255-3 Bettina Winzeler 1 , Nicole Cesana-Nigro 1 , Julie Refardt 1 , Deborah R Vogt 2 , Cornelia Imber 1 , Benedict Morin 1 , Milica Popovic 1 , Michelle Steinmetz 1 , Clara O Sailer 1 , Gabor Szinnai 3 , Irina Chifu 4 , Martin Fassnacht 5 , Mirjam Christ-Crain 1
Affiliation
|
BACKGROUND
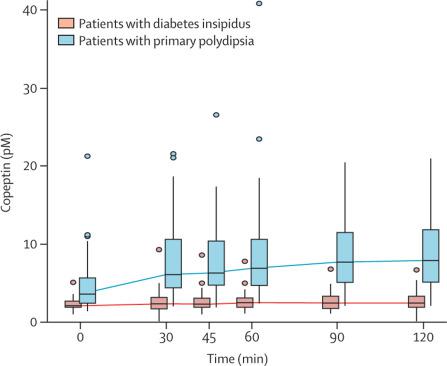
Differential diagnosis of diabetes insipidus is challenging. The most reliable approach is hypertonic saline-stimulated copeptin measurements. However, this test is based on the induction of hypernatraemia and requires close monitoring of plasma sodium concentrations. Arginine-stimulated copeptin measurements might provide an alternative, simple, and safe test.
METHODS
In this prospective diagnostic study, we recruited a development cohort from University Hospital Basel, Basel, Switzerland, and a validation cohort from five centres in Basel, Aarau, Luzern, Bern, and St Gallen, Switzerland, and the University Hospital Würzburg, Würzburg, Germany. For both cohorts, patients were eligible for inclusion if they were aged 18 years or older, were newly referred with polyuria (>50 mL/kg bodyweight per day) or had a known diagnosis of central diabetes insipidus or primary polydipsia. We also recruited a comparator cohort of healthy controls in parallel to each cohort, comprising adults (aged 18 years and older, with normal drinking habits, and no history of polyuria) and children who underwent arginine stimulation to diagnose growth hormone deficiency (children were only included in the comparator cohort to the development cohort as proof of concept). Patients and healthy controls underwent arginine stimulation with measurement of plasma copeptin at baseline and 30, 45, 60, 90, and 120 min. The primary objective in the development cohort was to determine the diagnostic accuracy of plasma copeptin concentrations to discriminate between diabetes insipidus and primary polydipsia, and in the validation cohort was to confirm those results. Adverse effects of the test were monitored in all participants, with tolerability of the test rated using a visual analogue scale (VAS) that ranged from no (0) to maximum (10) discomfort. This trial is registered with ClinicalTrials.gov, number NCT00757276.
FINDINGS
Between May 24, 2013, and Jan 11, 2017, 52 patients were enrolled in the development cohort (12 [23%] with complete diabetes insipidus, nine [17%] with partial diabetes insipidus, and 31 [60%] with primary polydipsia) alongside 20 healthy adults and 42 child controls. Between Oct 24, 2017, and June 27, 2018, 46 patients were enrolled in the validation cohort (12 [26%] with complete diabetes insipidus, seven [15%] with partial diabetes insipidus, and 27 [59%] with primary polydipsia) alongside 30 healthy adult controls (two patients in this cohort were excluded from the main analysis because of early vomiting during the test). In the pooled patient and control datasets, median arginine-stimulated copeptin concentrations increased in healthy adult controls (from 5·2 pM [IQR 3·3-10·9] to a maximum of 9·8 pM [6·4-19·6]) and in participants with primary polydipsia (from 3·6 pM [IQR 2·4-5·7] to a maximum of 7·9 pM [5·1-11·8]), but only minimally in those with diabetes insipidus (2·1 pM [IQR 1·9-2·7] to a maximum of 2·5 pM [1·9-3·1]). In the development cohort, a cutoff of 3·5 pM at 60 min provided the highest diagnostic accuracy of 94% (95% CI 84-98). The accuracy of this cutoff in the validation cohort was 86% (95% CI 73-94). By pooling the data from both cohorts, an optimal accuracy of 93% (95% CI 86-97) was reached at a cutoff of 3·8 pM copeptin at 60 min (sensitivity 93%, 95% CI 86-98; specificity 92%, 95% CI 84-100). The test was safe and well tolerated, with median VAS scores of 3·5 (IQR 2-4) in patients with diabetes insipidus, 3 (2-4) in those with primary polydipsia, 1 (1-3) in healthy adults, and 1 (0-5) in healthy children in the pooled participant dataset.
INTERPRETATION
Arginine-stimulated copeptin measurements are an innovative test for diabetes insipidus with high diagnostic accuracy, and could be a simplified, novel, and safe diagnostic approach to diabetes insipidus in clinical practice.
FUNDING
Swiss National Science Foundation and University Hospital Basel.
中文翻译:

精氨酸刺激的肽素测定对尿崩症的鉴别诊断:一项前瞻性诊断研究。
背景技术尿崩症的鉴别诊断具有挑战性。最可靠的方法是高渗盐水刺激的肽素测量。但是,该测试基于高钠血症的诱导,需要密切监测血浆钠浓度。精氨酸刺激的肽素测量可以提供一种替代,简单和安全的测试。方法在这项前瞻性诊断研究中,我们从瑞士巴塞尔的巴塞尔大学医院招募了一个开发队列,并从瑞士的巴塞尔,阿劳,卢塞恩,伯尔尼和圣加仑的五个中心以及维尔茨堡的维尔茨堡大学医院招募了一个验证队列。 , 德国。对于这两个队列,如果患者年龄在18岁或18岁以上,并且刚被转诊患有多尿症(> 每天50 mL / kg体重)或已知诊断为中枢性尿崩症或原发性多饮症。我们还招募了一个与对照组相对应的健康对照者队列,其中包括成年人(18岁及以上,有正常的饮酒习惯,无多尿史)和接受精氨酸刺激以诊断生长激素缺乏的儿童(仅儿童)。包括在比较队列中,也包括在开发队列中,以作为概念验证)。患者和健康对照组在基线以及30、45、60、90和120分钟时通过测量血浆肽素进行精氨酸刺激。这项研究的主要目的是确定血浆肽素浓度的诊断准确性,以区分尿崩症和原发性多饮症,在验证队列中是要确认那些结果。在所有参与者中监测测试的不良反应,并使用视觉模拟量表(VAS)对测试的耐受性进行评估,其评估范围为无不适(0)至最大不适(10)。该试验已在ClinicalTrials.gov上注册,编号为NCT00757276。结果在2013年5月24日至2017年1月11日之间,有52例患者入选了发育队列(12例[23%]患有完全尿崩症,9例[17%]患有部分尿崩症和31例[60%]原发性糖尿病)多动症)以及20名健康的成年人和42名儿童对照。在2017年10月24日至2018年6月27日之间,有46例患者入选验证队列(12例[26%]患有完全尿崩症,7例[15%]患有部分尿崩症,27例[59%]原发性多饮症)与30名健康成人对照(该队列中的2例患者由于测试期间的早期呕吐而被排除在主要分析之外)。在合并的患者和对照数据集中,健康成年人对照中精氨酸刺激的肽素浓度中位数增加(从5·2 pM [IQR 3·3-10·9]到最大9·8 pM [6·4-19· 6])和原发性多视症的参与者(从3·6 pM [IQR 2·4-5·7]到最大7·9 pM [5·1-11·8]),但在那些患有初发性多视的参与者中则很少尿崩症(2·1 pM [IQR 1·9-2·7]至最大2·5 pM [1·9-3·1])。在该开发队列中,在60分钟时达到3·5 pM的临界值提供了94%(95%CI 84-98)的最高诊断准确性。验证队列中此临界值的准确性为86%(95%CI 73-94)。通过汇总两个同类群组的数据,在60分钟时截止3·8 pM copeptin时,最佳准确度达到93%(95%CI 86-97)(灵敏度93%,95%CI 86-98;特异性92%,95%CI 84-100 )。该测试是安全且耐受性良好的,尿崩症患者的VAS评分中位数为3·5(IQR 2-4),原发性多视症患者的VAS评分中位数为3(2-4),健康成年人为1(1-3),在合并的参与者数据集中,健康儿童的得分为1(0-5)。解释精氨酸刺激的肽素测量是对尿崩症的一种创新测试,具有很高的诊断准确性,在临床实践中可能是一种简化,新颖且安全的对尿崩症的诊断方法。资助瑞士国家科学基金会和巴塞尔大学医院。该测试是安全且耐受性良好的,尿崩症患者的VAS评分中位数为3·5(IQR 2-4),原发性多视症患者的VAS评分中位数为3(2-4),健康成年人为1(1-3),在合并的参与者数据集中,健康儿童的得分为1(0-5)。解释精氨酸刺激的肽素测量是对尿崩症的一种创新测试,具有很高的诊断准确性,在临床实践中可能是一种简化,新颖且安全的对尿崩症的诊断方法。资助瑞士国家科学基金会和巴塞尔大学医院。该测试是安全且耐受性良好的,尿崩症患者的VAS评分中位数为3·5(IQR 2-4),原发性多视症患者的VAS评分中位数为3(2-4),健康成年人为1(1-3),在合并的参与者数据集中,健康儿童的得分为1(0-5)。解释精氨酸刺激的肽素测量是一种对尿崩症的创新测试,具有很高的诊断准确性,在临床实践中可能是一种简化,新颖,安全的对尿崩症的诊断方法。资助瑞士国家科学基金会和巴塞尔大学医院。解释精氨酸刺激的肽素测量是一种对尿崩症的创新测试,具有很高的诊断准确性,在临床实践中可能是一种简化,新颖,安全的对尿崩症的诊断方法。资助瑞士国家科学基金会和巴塞尔大学医院。解释精氨酸刺激的肽素测量是一种对尿崩症的创新测试,具有很高的诊断准确性,在临床实践中可能是一种简化,新颖,安全的对尿崩症的诊断方法。资助瑞士国家科学基金会和巴塞尔大学医院。
更新日期:2019-08-16
中文翻译:

精氨酸刺激的肽素测定对尿崩症的鉴别诊断:一项前瞻性诊断研究。
背景技术尿崩症的鉴别诊断具有挑战性。最可靠的方法是高渗盐水刺激的肽素测量。但是,该测试基于高钠血症的诱导,需要密切监测血浆钠浓度。精氨酸刺激的肽素测量可以提供一种替代,简单和安全的测试。方法在这项前瞻性诊断研究中,我们从瑞士巴塞尔的巴塞尔大学医院招募了一个开发队列,并从瑞士的巴塞尔,阿劳,卢塞恩,伯尔尼和圣加仑的五个中心以及维尔茨堡的维尔茨堡大学医院招募了一个验证队列。 , 德国。对于这两个队列,如果患者年龄在18岁或18岁以上,并且刚被转诊患有多尿症(> 每天50 mL / kg体重)或已知诊断为中枢性尿崩症或原发性多饮症。我们还招募了一个与对照组相对应的健康对照者队列,其中包括成年人(18岁及以上,有正常的饮酒习惯,无多尿史)和接受精氨酸刺激以诊断生长激素缺乏的儿童(仅儿童)。包括在比较队列中,也包括在开发队列中,以作为概念验证)。患者和健康对照组在基线以及30、45、60、90和120分钟时通过测量血浆肽素进行精氨酸刺激。这项研究的主要目的是确定血浆肽素浓度的诊断准确性,以区分尿崩症和原发性多饮症,在验证队列中是要确认那些结果。在所有参与者中监测测试的不良反应,并使用视觉模拟量表(VAS)对测试的耐受性进行评估,其评估范围为无不适(0)至最大不适(10)。该试验已在ClinicalTrials.gov上注册,编号为NCT00757276。结果在2013年5月24日至2017年1月11日之间,有52例患者入选了发育队列(12例[23%]患有完全尿崩症,9例[17%]患有部分尿崩症和31例[60%]原发性糖尿病)多动症)以及20名健康的成年人和42名儿童对照。在2017年10月24日至2018年6月27日之间,有46例患者入选验证队列(12例[26%]患有完全尿崩症,7例[15%]患有部分尿崩症,27例[59%]原发性多饮症)与30名健康成人对照(该队列中的2例患者由于测试期间的早期呕吐而被排除在主要分析之外)。在合并的患者和对照数据集中,健康成年人对照中精氨酸刺激的肽素浓度中位数增加(从5·2 pM [IQR 3·3-10·9]到最大9·8 pM [6·4-19· 6])和原发性多视症的参与者(从3·6 pM [IQR 2·4-5·7]到最大7·9 pM [5·1-11·8]),但在那些患有初发性多视的参与者中则很少尿崩症(2·1 pM [IQR 1·9-2·7]至最大2·5 pM [1·9-3·1])。在该开发队列中,在60分钟时达到3·5 pM的临界值提供了94%(95%CI 84-98)的最高诊断准确性。验证队列中此临界值的准确性为86%(95%CI 73-94)。通过汇总两个同类群组的数据,在60分钟时截止3·8 pM copeptin时,最佳准确度达到93%(95%CI 86-97)(灵敏度93%,95%CI 86-98;特异性92%,95%CI 84-100 )。该测试是安全且耐受性良好的,尿崩症患者的VAS评分中位数为3·5(IQR 2-4),原发性多视症患者的VAS评分中位数为3(2-4),健康成年人为1(1-3),在合并的参与者数据集中,健康儿童的得分为1(0-5)。解释精氨酸刺激的肽素测量是对尿崩症的一种创新测试,具有很高的诊断准确性,在临床实践中可能是一种简化,新颖且安全的对尿崩症的诊断方法。资助瑞士国家科学基金会和巴塞尔大学医院。该测试是安全且耐受性良好的,尿崩症患者的VAS评分中位数为3·5(IQR 2-4),原发性多视症患者的VAS评分中位数为3(2-4),健康成年人为1(1-3),在合并的参与者数据集中,健康儿童的得分为1(0-5)。解释精氨酸刺激的肽素测量是对尿崩症的一种创新测试,具有很高的诊断准确性,在临床实践中可能是一种简化,新颖且安全的对尿崩症的诊断方法。资助瑞士国家科学基金会和巴塞尔大学医院。该测试是安全且耐受性良好的,尿崩症患者的VAS评分中位数为3·5(IQR 2-4),原发性多视症患者的VAS评分中位数为3(2-4),健康成年人为1(1-3),在合并的参与者数据集中,健康儿童的得分为1(0-5)。解释精氨酸刺激的肽素测量是一种对尿崩症的创新测试,具有很高的诊断准确性,在临床实践中可能是一种简化,新颖,安全的对尿崩症的诊断方法。资助瑞士国家科学基金会和巴塞尔大学医院。解释精氨酸刺激的肽素测量是一种对尿崩症的创新测试,具有很高的诊断准确性,在临床实践中可能是一种简化,新颖,安全的对尿崩症的诊断方法。资助瑞士国家科学基金会和巴塞尔大学医院。解释精氨酸刺激的肽素测量是一种对尿崩症的创新测试,具有很高的诊断准确性,在临床实践中可能是一种简化,新颖,安全的对尿崩症的诊断方法。资助瑞士国家科学基金会和巴塞尔大学医院。



























 京公网安备 11010802027423号
京公网安备 11010802027423号