Kidney International ( IF 19.6 ) Pub Date : 2019-07-10 , DOI: 10.1016/j.kint.2019.06.014 Dale R Abrahamson 1 , Brooke M Steenhard 1 , Larysa Stroganova 1 , Adrian Zelenchuk 1 , Patricia L St John 1 , Margaret G Petroff 2 , Manuel Patarroyo 3 , Dorin Bogdan Borza 4
|
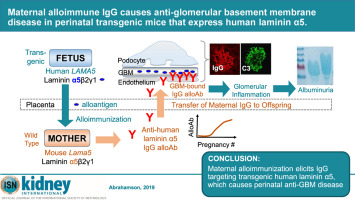
Mammalian immune systems are not mature until well after birth. However, transfer of maternal IgG to the fetus and newborn usually provides immunoprotection from infectious diseases. IgG transfer occurs before birth in humans across the placenta and continues after birth across the intestine in many mammalian species, including rodents. Transfer, which is mediated by the neonatal IgG Fc receptor, occurs by transcytosis across placental syncytiotrophoblasts and intestinal epithelium. Although maternal IgG is generally beneficial, harmful maternal allo- and autoantibodies can also be transferred to the fetus/infant, resulting in serious disease. To test this we generated transgenic mice that widely express human laminin α5 in their basement membranes. When huLAMA5 transgenic males were crossed with wild-type females, there was a maternal anti-human laminin α5 immune response. Maternal IgG alloantibody crossed the yolk sac and post-natal intestine in vivo and bound in bright, linear patterns to kidney glomerular basement membranes of transgenic fetuses/neonates but not those of wild-type siblings. By postnatal day 18, most transgenic mice were proteinuric, had glomerular C3 deposits and inflammatory cell infiltrates, thickened and split glomerular basement membranes, and podocyte foot process effacement. Thus, our novel model of perinatal anti-glomerular basement membrane disease may prove useful for studying pediatric glomerulopathies, formation of the fetomaternal interface, and maternal alloimmunization.
中文翻译:

母源同种免疫IgG在表达人层粘连蛋白α5的围产期转基因小鼠中引起抗肾小球基底膜疾病。
哺乳动物的免疫系统直到出生后才成熟。但是,将母体IgG转移至胎儿和新生儿通常可提供针对传染病的免疫保护。IgG的转移发生在人类出生前穿过胎盘,并在出生后在整个哺乳动物体内持续扩散,包括啮齿类在内的许多哺乳动物物种。由新生IgG Fc受体介导的转移是通过跨胎盘合体滋养层细胞和肠上皮的胞吞作用发生的。尽管母体IgG通常是有益的,但有害的母体同种抗体和自身抗体也可以转移至胎儿/婴儿,从而导致严重的疾病。为了测试这一点,我们生成了在基膜中广泛表达人层粘连蛋白α5的转基因小鼠。当胡LAMA5转基因雄性与野生型雌性杂交,母体产生了抗人层粘连蛋白α5的免疫反应。母体IgG同种异体抗体在 体内穿过卵黄囊和产后肠道,并以明亮的线性模式与转基因胎儿/新生儿的肾小球基底膜结合,但与野生型同胞的肾脏肾小球基底膜结合。出生后第18天,大多数转基因小鼠具有蛋白尿,肾小球C3沉积和炎性细胞浸润,肾小球基底膜增厚和裂开,足细胞足突消失。因此,我们的围产期抗肾小球基底膜疾病的新模型可能被证明对研究小儿肾小球病变,胎儿母体界面的形成和母体同种免疫非常有用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号