当前位置:
X-MOL 学术
›
Cancer Gene Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
TBL1XR1 is involved in c-Met-mediated tumorigenesis of human nonsmall cell lung cancer.
Cancer Gene Therapy ( IF 6.4 ) Pub Date : 2019-06-27 , DOI: 10.1038/s41417-019-0111-0 Tiewa Zhang 1 , Cheng Liu 2 , Yan Yu 3 , Jianxiong Geng 3 , Qingwei Meng 3 , Shanqi Xu 3 , Fengrui Zhou 3 , Yingying Chen 4 , Shi Jin 5 , Jing Shen 6 , Bo Pan 3 , Fanling Meng 7 , Fang Liu 3
Cancer Gene Therapy ( IF 6.4 ) Pub Date : 2019-06-27 , DOI: 10.1038/s41417-019-0111-0 Tiewa Zhang 1 , Cheng Liu 2 , Yan Yu 3 , Jianxiong Geng 3 , Qingwei Meng 3 , Shanqi Xu 3 , Fengrui Zhou 3 , Yingying Chen 4 , Shi Jin 5 , Jing Shen 6 , Bo Pan 3 , Fanling Meng 7 , Fang Liu 3
Affiliation
|
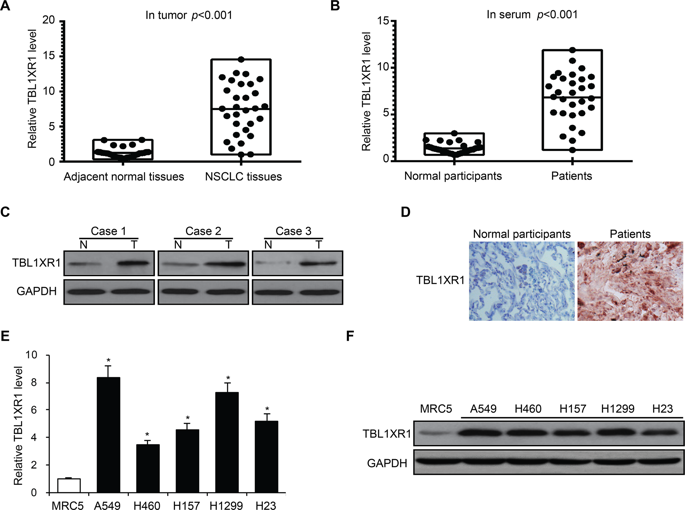
Nonsmall cell lung carcinoma (NSCLC) contributes to the highest number of cancer deaths globally. Metastases and chemoresistance are two major confounders to the treatment efficacy in NSCLC. Transducin (β)-like 1 X-linked receptor 1 (TBL1XR1) has been associated with high rates of metastases in breast, gastric, and stomach cancers. However, the role of TBL1XR1 in lung cancers remains underexplored. We selected matched and cancerous lung tissues to establish the upregulation of TBL1XR1. Using in vitro assays, we assessed the influence of TBL1XR1 on various cancer phenotypes, namely cell proliferation, chemoresistance, invasion, and metastases in a CRISPR-Cas9-mediated knock out model (A549 cells), and H460 cell lines overexpressing TBL1XR1. We found that TBL1XR1 is overexpressed in NSCLC tissue and patient sera in comparison to paired adjacent normal tissue. Overexpression of TBL1XR1 in NSCLC cell lines mediates cell survival, proliferation, and metastases. TBL1XR1 was found to regulate MEK and Akt pathways through their master regulator c-Met. We observed that activation of c-Met is downregulated in the absence of TBL1XR1. Our study strengthens the contention that TBL1XR1 is a biomarker for prognosis of NSCLC. It may also be considered as an adjunct or core therapeutic target to overcome cisplatin resistance in lung cancers.
中文翻译:

TBL1XR1参与人类非小细胞肺癌的c-Met介导的肿瘤发生。
非小细胞肺癌(NSCLC)是全球癌症死亡人数最多的原因。转移和化学耐药是非小细胞肺癌治疗疗效的两个主要混杂因素。类转导蛋白(β)的1 X连锁受体1(TBL1XR1)与乳腺癌,胃癌和胃癌的高转移率相关。但是,TBL1XR1在肺癌中的作用仍未得到充分研究。我们选择了匹配的癌性肺组织以建立TBL1XR1的上调。使用体外测定,我们评估了TBL1XR1对各种癌症表型的影响,即CRISPR-Cas9介导的敲除模型(A549细胞)和过表达TBL1XR1的H460细胞系中的细胞增殖,化学抗性,侵袭和转移。我们发现与配对的相邻正常组织相比,TBL1XR1在NSCLC组织和患者血清中过表达。TCL1XR1在NSCLC细胞系中的过表达介导细胞存活,增殖和转移。发现TBL1XR1通过其主调节剂c-Met调节MEK和Akt途径。我们观察到在没有TBL1XR1的情况下c-Met的激活被下调。我们的研究加强了关于TBL1XR1是非小细胞肺癌预后的生物标志物的争论。它也可以被视为克服肺癌中顺铂耐药性的辅助或核心治疗靶标。我们观察到在没有TBL1XR1的情况下c-Met的激活被下调。我们的研究加强了关于TBL1XR1是非小细胞肺癌预后的生物标志物的争论。它也可以被视为克服肺癌中顺铂耐药性的辅助或核心治疗靶标。我们观察到在没有TBL1XR1的情况下c-Met的激活被下调。我们的研究加强了关于TBL1XR1是非小细胞肺癌预后的生物标志物的争论。它也可以被视为克服肺癌中顺铂耐药性的辅助或核心治疗靶标。
更新日期:2019-11-18
中文翻译:

TBL1XR1参与人类非小细胞肺癌的c-Met介导的肿瘤发生。
非小细胞肺癌(NSCLC)是全球癌症死亡人数最多的原因。转移和化学耐药是非小细胞肺癌治疗疗效的两个主要混杂因素。类转导蛋白(β)的1 X连锁受体1(TBL1XR1)与乳腺癌,胃癌和胃癌的高转移率相关。但是,TBL1XR1在肺癌中的作用仍未得到充分研究。我们选择了匹配的癌性肺组织以建立TBL1XR1的上调。使用体外测定,我们评估了TBL1XR1对各种癌症表型的影响,即CRISPR-Cas9介导的敲除模型(A549细胞)和过表达TBL1XR1的H460细胞系中的细胞增殖,化学抗性,侵袭和转移。我们发现与配对的相邻正常组织相比,TBL1XR1在NSCLC组织和患者血清中过表达。TCL1XR1在NSCLC细胞系中的过表达介导细胞存活,增殖和转移。发现TBL1XR1通过其主调节剂c-Met调节MEK和Akt途径。我们观察到在没有TBL1XR1的情况下c-Met的激活被下调。我们的研究加强了关于TBL1XR1是非小细胞肺癌预后的生物标志物的争论。它也可以被视为克服肺癌中顺铂耐药性的辅助或核心治疗靶标。我们观察到在没有TBL1XR1的情况下c-Met的激活被下调。我们的研究加强了关于TBL1XR1是非小细胞肺癌预后的生物标志物的争论。它也可以被视为克服肺癌中顺铂耐药性的辅助或核心治疗靶标。我们观察到在没有TBL1XR1的情况下c-Met的激活被下调。我们的研究加强了关于TBL1XR1是非小细胞肺癌预后的生物标志物的争论。它也可以被视为克服肺癌中顺铂耐药性的辅助或核心治疗靶标。



























 京公网安备 11010802027423号
京公网安备 11010802027423号