Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pexidartinib versus placebo for advanced tenosynovial giant cell tumour (ENLIVEN): a randomised phase 3 trial.
The Lancet ( IF 168.9 ) Pub Date : 2019-06-19 , DOI: 10.1016/s0140-6736(19)30764-0 William D Tap 1 , Hans Gelderblom 2 , Emanuela Palmerini 3 , Jayesh Desai 4 , Sebastian Bauer 5 , Jean-Yves Blay 6 , Thierry Alcindor 7 , Kristen Ganjoo 8 , Javier Martín-Broto 9 , Christopher W Ryan 10 , David M Thomas 11 , Charles Peterfy 12 , John H Healey 1 , Michiel van de Sande 2 , Heather L Gelhorn 13 , Dale E Shuster 14 , Qiang Wang 14 , Antoine Yver 14 , Henry H Hsu 15 , Paul S Lin 15 , Sandra Tong-Starksen 15 , Silvia Stacchiotti 16 , Andrew J Wagner 17 ,
中文翻译:

Pexidartinib与安慰剂治疗晚期腱鞘巨细胞瘤(ENLIVEN):一项随机的3期临床试验。
更新日期:2019-08-09
The Lancet ( IF 168.9 ) Pub Date : 2019-06-19 , DOI: 10.1016/s0140-6736(19)30764-0 William D Tap 1 , Hans Gelderblom 2 , Emanuela Palmerini 3 , Jayesh Desai 4 , Sebastian Bauer 5 , Jean-Yves Blay 6 , Thierry Alcindor 7 , Kristen Ganjoo 8 , Javier Martín-Broto 9 , Christopher W Ryan 10 , David M Thomas 11 , Charles Peterfy 12 , John H Healey 1 , Michiel van de Sande 2 , Heather L Gelhorn 13 , Dale E Shuster 14 , Qiang Wang 14 , Antoine Yver 14 , Henry H Hsu 15 , Paul S Lin 15 , Sandra Tong-Starksen 15 , Silvia Stacchiotti 16 , Andrew J Wagner 17 ,
Affiliation
|
Background
Tenosynovial giant cell tumour (TGCT), a rare, locally aggressive neoplasm, overexpresses colony-stimulating factor 1 (CSF1). Surgery is standard with no approved systemic therapy. We aimed to evaluate pexidartinib, a CSF1 receptor inhibitor, in patients with TGCT to provide them with a viable systemic treatment option, especially in cases that are not amenable to surgical resection.Methods
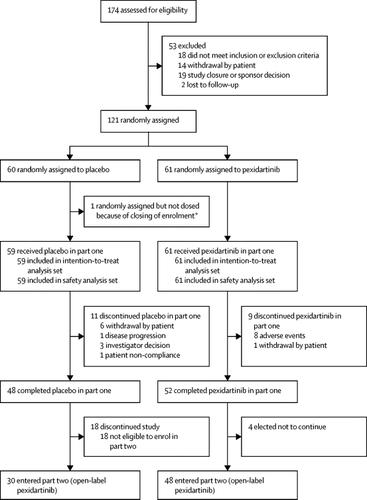
This phase 3 randomised trial had two parts. Part one was a double-blind study in which patients with symptomatic, advanced TGCT for whom surgery was not recommended were randomly assigned via an integrated web response system (1:1) to the pexidartinib or placebo group. Individuals in the pexidartinib group received a loading dose of 1000 mg pexidartinib per day orally (400 mg morning; 600 mg evening) for the first 2 weeks, followed by 800 mg per day (400 mg twice a day) for 22 weeks. Part two was an open-label study of pexidartinib for all patients. The primary endpoint, assessed in all intention-to-treat patients, was overall response at week 25, and was centrally reviewed by RECIST, version 1.1. Safety was analysed in all patients who received at least one dose of the study drug. This study is registered with , number .Findings
Between May 11, 2015, and Sept 30, 2016, of 174 patients assessed for eligibility, 120 patients were randomly assigned to, and received, pexidartinib (n=61) or placebo (n=59). There were 11 dropouts in the placebo group and nine in the pexidartinib group. Emergence of mixed or cholestatic hepatotoxicity caused the data monitoring committee to stop enrolment six patients short of target. The proportion of patients who achieved overall response was higher for pexidartinib than placebo at week 25 by RECIST (24 [39%] of 61 vs none of 59; absolute difference 39% [95% CI 27–53]; p<0·0001). Serious adverse events occurred in eight (13%) of 61 patients in the pexidartinib group and one (2%) of 59 patients in the placebo group. Hair colour changes (67%), fatigue (54%), aspartate aminotransferase increase (39%), nausea (38%), alanine aminotransferase increase (28%), and dysgeusia (25%) were the most frequent pexidartinib-associated adverse events. Three patients given pexidartinib had aminotransferase elevations three or more times the upper limit of normal with total bilirubin and alkaline phosphatase two or more times the upper limit of normal indicative of mixed or cholestatic hepatotoxicity, one lasting 7 months and confirmed by biopsy.Interpretation
Pexidartinib is the first systemic therapy to show a robust tumour response in TGCT with improved patient symptoms and functional outcomes; mixed or cholestatic hepatotoxicity is an identified risk. Pexidartinib could be considered as a potential treatment for TGCT associated with severe morbidity or functional limitations in cases not amenable to improvement with surgery.Funding
Daiichi Sankyo.中文翻译:

Pexidartinib与安慰剂治疗晚期腱鞘巨细胞瘤(ENLIVEN):一项随机的3期临床试验。



























 京公网安备 11010802027423号
京公网安备 11010802027423号