当前位置:
X-MOL 学术
›
Theranostics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Temporal inhibition of mouse mammary gland cancer metastasis by CORM-A1 and DETA/NO combination therapy.
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.31461 Kseniia Porshneva 1 , Diana Papiernik 1 , Mateusz Psurski 1 , Agnieszka Łupicka-Słowik 2 , Rafał Matkowski 3, 4 , Marcin Ekiert 3, 4 , Marcin Nowak 5 , Joanna Jarosz 1 , Joanna Banach 1 , Magdalena Milczarek 1 , Tomasz M Goszczyński 1 , Marcin Sieńczyk 2 , Joanna Wietrzyk 1
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.31461 Kseniia Porshneva 1 , Diana Papiernik 1 , Mateusz Psurski 1 , Agnieszka Łupicka-Słowik 2 , Rafał Matkowski 3, 4 , Marcin Ekiert 3, 4 , Marcin Nowak 5 , Joanna Jarosz 1 , Joanna Banach 1 , Magdalena Milczarek 1 , Tomasz M Goszczyński 1 , Marcin Sieńczyk 2 , Joanna Wietrzyk 1
Affiliation
|
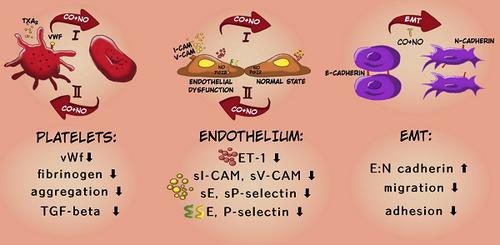
Carbon monoxide and nitric oxide are two of the most important vasoprotective mediators. Their downregulation observed during vascular dysfunction, which is associated with cancer progression, leads to uncontrolled platelet activation. Therefore, the aim of our studies was to improve vasoprotection and to decrease platelet activation during progression of mouse mammary gland cancer by concurrent use of CO and NO donors (CORM-A1 and DETA/NO, respectively). Methods: Mice injected intravenously with 4T1-luc2-tdTomato or orthotopically with 4T1 mouse mammary gland cancer cells were treated with CORM-A1 and DETA/NO. Ex vivo aggregation and activation of platelets were assessed in the blood of healthy donors and breast cancer patients. Moreover, we analyzed the compounds' direct effect on 4T1 mouse and MDA-MB-231 human breast cancer cells proliferation, adhesion and migration in vitro. Results: We have observed antimetastatic effect of combination therapy, which was only transient in orthotopic model. During early stages of tumor progression concurrent use of CORM-A1 and DETA/NO demonstrated vasoprotective ability (decreased endothelin-1, sICAM and sE-selectin plasma level) and downregulated platelets activation (decreased bound of fibrinogen and vWf to platelets) as well as inhibited EMT process. Combined treatment with CO and NO donors diminished adhesion and migration of breast cancer cells in vitro and inhibited aggregation as well as TGF-β release from breast cancer patients' platelets ex vivo. However, antimetastatic effect was not observed at a later stage of tumor progression which was accompanied by increased platelets activation and endothelial dysfunction related to a decrease of VASP level. Conclusion: The therapy was shown to have antimetastatic action and resulted in normalization of endothelial metabolism, diminution of platelet activation and inhibition of EMT process. The effect was more prominent during early stages of tumor dissemination. Such treatment could be applied to inhibit metastasis during the first stages of this process.
中文翻译:

CORM-A1和DETA / NO联合疗法对小鼠乳腺癌转移的时间抑制作用。
一氧化碳和一氧化氮是最重要的两种血管保护介质。在血管功能异常期间观察到的它们的下调,这与癌症的进展有关,导致不受控制的血小板活化。因此,我们研究的目的是通过同时使用CO和NO供体(分别为COM-A1和DETA / NO)来改善小鼠乳腺癌进展过程中的血管保护作用并减少血小板活化。方法:静脉注射4T1-luc2-tdTomato小鼠或原位注射4T1小鼠乳腺癌细胞,分别用CORM-A1和DETA / NO处理。在健康供体和乳腺癌患者的血液中评估了离体聚集和血小板活化。此外,我们分析了化合物的 直接作用于4T1小鼠和MDA-MB-231人乳腺癌细胞的体外增殖,粘附和迁移。结果:我们观察到联合疗法的抗转移作用,仅在原位模型中是短暂的。在肿瘤进展的早期阶段,同时使用CORM-A1和DETA / NO表现出血管保护能力(内皮素-1,sICAM和sE-选择素血浆水平降低)和血小板活化下调(纤维蛋白原和vWf与血小板的结合降低)以及抑制了EMT过程。CO和NO供体的联合治疗减少了乳腺癌细胞的体外粘附和迁移,并抑制了乳腺癌患者离体血小板的聚集以及TGF-β的释放。然而,在肿瘤进展的后期未观察到抗转移作用,其伴随着血小板活化增加和与VASP水平降低有关的内皮功能障碍。结论:该疗法具有抗转移作用,可导致内皮代谢正常化,血小板活化减少和EMT过程抑制。在肿瘤扩散的早期阶段,该作用更为显着。可以在该过程的最初阶段应用这种治疗来抑制转移。在肿瘤扩散的早期阶段,该作用更为显着。可以在该过程的最初阶段应用这种治疗来抑制转移。在肿瘤扩散的早期阶段,该作用更为显着。可以在该过程的最初阶段应用这种治疗来抑制转移。
更新日期:2019-01-01
中文翻译:

CORM-A1和DETA / NO联合疗法对小鼠乳腺癌转移的时间抑制作用。
一氧化碳和一氧化氮是最重要的两种血管保护介质。在血管功能异常期间观察到的它们的下调,这与癌症的进展有关,导致不受控制的血小板活化。因此,我们研究的目的是通过同时使用CO和NO供体(分别为COM-A1和DETA / NO)来改善小鼠乳腺癌进展过程中的血管保护作用并减少血小板活化。方法:静脉注射4T1-luc2-tdTomato小鼠或原位注射4T1小鼠乳腺癌细胞,分别用CORM-A1和DETA / NO处理。在健康供体和乳腺癌患者的血液中评估了离体聚集和血小板活化。此外,我们分析了化合物的 直接作用于4T1小鼠和MDA-MB-231人乳腺癌细胞的体外增殖,粘附和迁移。结果:我们观察到联合疗法的抗转移作用,仅在原位模型中是短暂的。在肿瘤进展的早期阶段,同时使用CORM-A1和DETA / NO表现出血管保护能力(内皮素-1,sICAM和sE-选择素血浆水平降低)和血小板活化下调(纤维蛋白原和vWf与血小板的结合降低)以及抑制了EMT过程。CO和NO供体的联合治疗减少了乳腺癌细胞的体外粘附和迁移,并抑制了乳腺癌患者离体血小板的聚集以及TGF-β的释放。然而,在肿瘤进展的后期未观察到抗转移作用,其伴随着血小板活化增加和与VASP水平降低有关的内皮功能障碍。结论:该疗法具有抗转移作用,可导致内皮代谢正常化,血小板活化减少和EMT过程抑制。在肿瘤扩散的早期阶段,该作用更为显着。可以在该过程的最初阶段应用这种治疗来抑制转移。在肿瘤扩散的早期阶段,该作用更为显着。可以在该过程的最初阶段应用这种治疗来抑制转移。在肿瘤扩散的早期阶段,该作用更为显着。可以在该过程的最初阶段应用这种治疗来抑制转移。


























 京公网安备 11010802027423号
京公网安备 11010802027423号