当前位置:
X-MOL 学术
›
Theranostics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
pH- and photothermal-driven multistage delivery nanoplatform for overcoming cancer drug resistance.
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.33958 Wenjing Huang 1 , Hao Zhao 1 , Jiangshan Wan 1 , Yang Zhou 1 , Qingbo Xu 1 , Yanbing Zhao 1, 2 , Xiangliang Yang 1, 2 , Lu Gan 1, 2
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.33958 Wenjing Huang 1 , Hao Zhao 1 , Jiangshan Wan 1 , Yang Zhou 1 , Qingbo Xu 1 , Yanbing Zhao 1, 2 , Xiangliang Yang 1, 2 , Lu Gan 1, 2
Affiliation
|
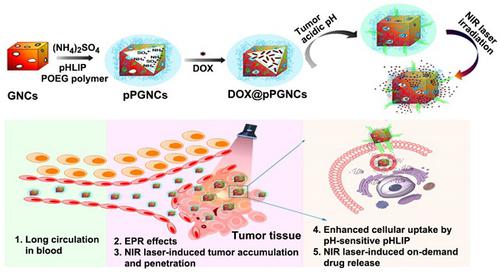
Reversing multidrug resistance (MDR) remains a big challenge in cancer therapy. Combining the hyperthermia and chemotherapy is a promising strategy for efficient cancer treatment with MDR reversal. Gold nanocages (GNCs) are an ideal photothermal (PTT)-chemotherapy integration platform due to their good photothermal conversion efficiency and the unique hollow interiors. However, insufficient tumor cell internalization and in vivo premature drug leakage restrict the anticancer activity of GNCs-based drug delivery systems. Methods: pH low insertion peptide (pHLIP)- and thermoresponsive poly(di(ethylene glycol) methyl ether methacrylate-co-oligo(ethylene glycol) methyl ether methacrylate) polymer-conjugated GNCs were rationally constructed to load anticancer drug doxorubicin (DOX@pPGNCs). Tumor acidic environment-responsive tumor cell internalization, and near-infrared (NIR) laser-induced tumor accumulation, penetration and on-demand drug release were systematically examined. Results: DOX@pPGNCs display good photothermal efficacy and thermoresponsive property. NIR laser irradiations at the tumor site significantly enhance tumor accumulation and penetration. Once DOX@pPGNCs reach the tumor site, the conformational transformation of pHLIP at the acidic tumor microenvironment contributes to the enhanced cellular internalization. Furthermore, NIR laser-triggered photothermal effects induce the shrinkage of thermoresponsive polymer, resulting in the opening of the pores of GNCs and a rapid intracellular DOX release to the nuclei. DOX@pPGNCs exhibit synergistic antitumor effect with MDR reversal in vitro and in vivo. Conclusion: DOX@pPGNCs present strong potential to overcome MDR in cancer.
中文翻译:

pH和光热驱动的多阶段递送纳米平台可克服癌症的耐药性。
逆转多药耐药性(MDR)仍然是癌症治疗中的一大挑战。热疗和化学疗法的结合是一种有效的癌症治疗方法,可逆转MDR。金纳米笼(GNC)由于其良好的光热转换效率和独特的中空内部结构,是理想的光热(PTT)-化学疗法集成平台。然而,不足的肿瘤细胞内在化和体内过早的药物泄漏限制了基于GNCs的药物递送系统的抗癌活性。方法:合理构建pH低插入肽(pHLIP)和热响应性聚(二(乙二醇)甲基丙烯酸二甲酯-共聚(乙二醇)甲基醚甲基丙烯酸甲酯)聚合物偶联的GNC,以加载抗癌药阿霉素(DOX @ pPGNCs) )。肿瘤酸性环境反应性肿瘤细胞内在化,并系统地检查了近红外(NIR)激光诱导的肿瘤蓄积,渗透和按需药物释放。结果:DOX @ pPGNCs显示出良好的光热功效和热响应性。在肿瘤部位的NIR激光辐照显着增强了肿瘤的积累和渗透。一旦DOX @ pPGNCs到达肿瘤部位,酸性肿瘤微环境中pHLIP的构象转化就有助于增强细胞内在化。此外,NIR激光触发的光热效应引起热响应性聚合物的收缩,从而导致GNC孔的开放和细胞内DOX迅速释放到核中。DOX @ pPGNCs在体外和体内均表现出与MDR逆转的协同抗肿瘤作用。结论:DOX @ pPGNCs具有克服癌症中MDR的强大潜力。
更新日期:2019-01-01
中文翻译:

pH和光热驱动的多阶段递送纳米平台可克服癌症的耐药性。
逆转多药耐药性(MDR)仍然是癌症治疗中的一大挑战。热疗和化学疗法的结合是一种有效的癌症治疗方法,可逆转MDR。金纳米笼(GNC)由于其良好的光热转换效率和独特的中空内部结构,是理想的光热(PTT)-化学疗法集成平台。然而,不足的肿瘤细胞内在化和体内过早的药物泄漏限制了基于GNCs的药物递送系统的抗癌活性。方法:合理构建pH低插入肽(pHLIP)和热响应性聚(二(乙二醇)甲基丙烯酸二甲酯-共聚(乙二醇)甲基醚甲基丙烯酸甲酯)聚合物偶联的GNC,以加载抗癌药阿霉素(DOX @ pPGNCs) )。肿瘤酸性环境反应性肿瘤细胞内在化,并系统地检查了近红外(NIR)激光诱导的肿瘤蓄积,渗透和按需药物释放。结果:DOX @ pPGNCs显示出良好的光热功效和热响应性。在肿瘤部位的NIR激光辐照显着增强了肿瘤的积累和渗透。一旦DOX @ pPGNCs到达肿瘤部位,酸性肿瘤微环境中pHLIP的构象转化就有助于增强细胞内在化。此外,NIR激光触发的光热效应引起热响应性聚合物的收缩,从而导致GNC孔的开放和细胞内DOX迅速释放到核中。DOX @ pPGNCs在体外和体内均表现出与MDR逆转的协同抗肿瘤作用。结论:DOX @ pPGNCs具有克服癌症中MDR的强大潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号