npj Vaccines ( IF 9.2 ) Pub Date : 2019-05-29 , DOI: 10.1038/s41541-019-0114-8 Min Z. Levine , Crystal Holiday , Stacie Jefferson , F. Liaini Gross , Feng Liu , Sheng Li , Damien Friel , Philippe Boutet , Bruce L. Innis , Corey P. Mallett , Terrence M. Tumpey , James Stevens , Jacqueline M. Katz
|
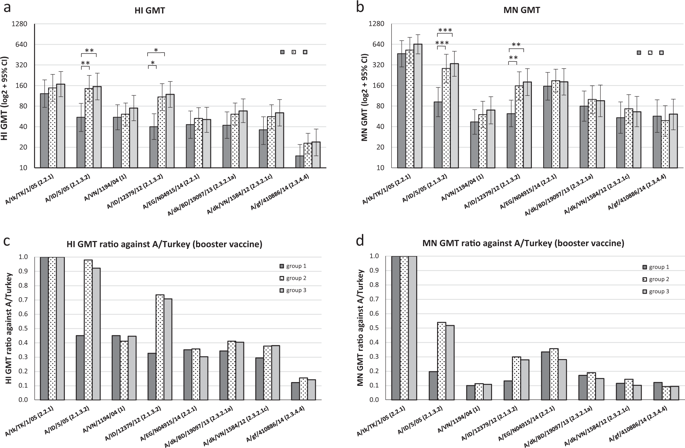
Highly pathogenic avian influenza (HPAI) A(H5Nx) viruses continue to pose a pandemic threat. US national vaccine stockpiles are a cornerstone of the influenza pandemic preparedness plans. However, continual genetic and antigenic divergence of A(H5Nx) viruses requires the development of effective vaccination strategies using stockpiled vaccines and adjuvants for pandemic preparedness. Human sera collected from healthy adults who received either homologous (2 doses of a AS03A-adjuvanted A/turkey/Turkey/1/2005, A/Turkey), or heterologous (primed with AS03A-adjuvanted A/Indonesia/5/2005, A/Indo, followed by A/Turkey boost) prime-boost vaccination regimens were analyzed by hemagglutination inhibition and microneutralization assays against 8 wild-type HPAI A(H5Nx) viruses from 6 genetic clades. Molecular, structural and antigenic features of the A(H5Nx) viruses that could influence the cross-clade antibody responses were also explored. Compared with homologous prime-boost vaccinations, priming with a clade 2.1.3.2 antigen (A/Indo) followed by one booster dose of a clade 2.2.1 antigen (A/Turkey) administered 18 months apart did not compromise the antibody responses to the booster vaccine (A/Turkey), it also broadened the cross-clade antibody responses to several antigenically drifted variants from 6 heterologous clades, including an antigenically distant A(H5N8) virus (A/gyrfalcon/Washington/410886/2014, clade 2.3.4.4) that caused recent outbreaks in US poultry. The magnitude and breadth of the cross-clade antibody responses against emerging HPAI A(H5Nx) viruses are associated with genetic, structural and antigenic differences from the vaccine viruses and enhanced by the inclusion of an adjuvant. Heterologous prime-boost vaccination with AS03A adjuvanted vaccine offers a vaccination strategy to use existing stockpiled vaccines for pandemic preparedness against new emerging HPAI A(H5Nx) viruses.
中文翻译:

A(H5N1)大流行性流感疫苗的异源初免-加强比同源初免-加强诱导广泛的交叉抗体反应
高致病性禽流感(HPAI)A(H5Nx)病毒继续构成大流行威胁。美国国家疫苗库存是流感大流行防范计划的基石。但是,A(H5Nx)病毒的持续遗传和抗原分歧要求开发使用储备疫苗和佐剂的有效疫苗接种策略,以防大流行。从健康成年人中收集的人类血清,这些成年人接受了同源(2剂AS03 A佐剂化的A / turkey / Turkey / 1/2005,A / Turkey)或异源性(以AS03 A引发)辅助佐剂的A /印度尼西亚/ 5/2005,A /印度,然后是A /土耳其加强)通过血凝抑制和微中和试验对来自6个基因进化枝的8种野生型HPAI A(H5Nx)病毒进行了初次加强免疫接种方案的分析。还探讨了可能影响交叉抗体反应的A(H5Nx)病毒的分子,结构和抗原特性。与同源初免-加强疫苗接种相比,先后进行进化枝2.1.3.2抗原(A / Indo)和一剂加强剂量的进化枝2.2.1抗原(A / Turkey)的疫苗接种,间隔18个月并不影响抗体对抗体的反应。增强疫苗(A / Turkey),它还扩展了跨交叉抗体对来自6个异源进化枝的几种抗原性漂移变体的反应,包括造成美国家禽近期暴发的抗原性遥远的A(H5N8)病毒(A / gyrfalcon / Washington / 410886/2014,进化枝2.3.4.4)。针对新兴的HPAI A(H5Nx)病毒的交叉抗体应答的强度和广度与疫苗病毒的遗传,结构和抗原性差异相关,并通过包含佐剂而得到增强。用AS03进行异源初免接种一佐剂疫苗提供免疫接种策略,以利用现有储存的疫苗针对新出现的高致病性禽流感A(H5Nx)病毒大流行的准备。


























 京公网安备 11010802027423号
京公网安备 11010802027423号