Cell Discovery ( IF 33.5 ) Pub Date : 2019-05-28 , DOI: 10.1038/s41421-019-0101-2 Zeyuan Guan , Kai Pei , Jing Wang , Yongqing Cui , Xiang Zhu , Xiang Su , Yuanbao Zhou , Delin Zhang , Chun Tang , Ping Yin , Zhu Liu , Tingting Zou
|
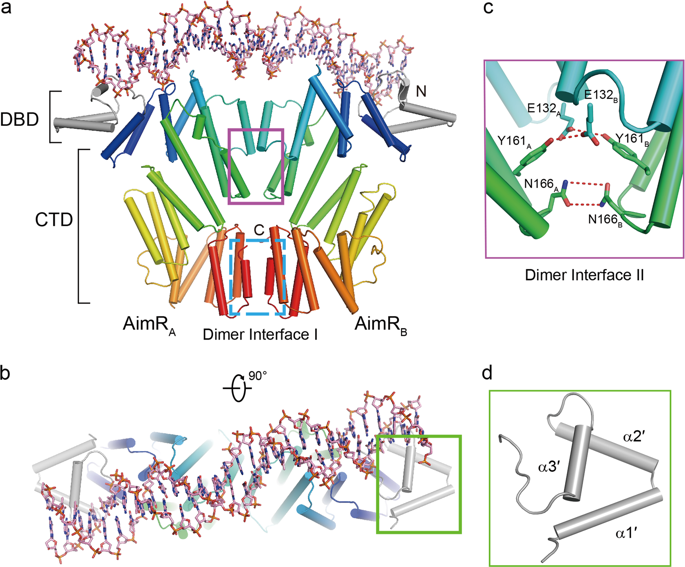
A newly identified arbitrium communication system regulates the lysis-to-lysogeny decision in a Bacillus bacteriophage. This system contains an arbitrium hexapeptide as a signal, the cellular receptor AimR, and the lysogenic negative regulator AimX. AimR specifically targets the downstream DNA to activate aimX gene expression. The arbitrium peptide binds to AimR, inhibiting its DNA-binding to promote phage lysogeny. Recently, we and other groups have elucidated how arbitrium peptide sensed by AimR. However, the molecular mechanisms of DNA recognition by AimR and the regulation of its DNA-binding activity by the peptide remain largely unknown. Here, we report the crystal structure of the AimR–DNA complex at 2.1 Å resolution. The N-terminal HTH motif recognizes the palindromic DNA sequence, buttressed by interactions between positively charged residues and the DNA phosphate groups. The DNA-bound AimR assembles a more closed dimer than the peptide-bound form. Single-molecule FRET and crosslinking assays revealed that the AimR protein samples both open and closed conformations in solution. Arbitrium peptide binding induces a closed-to-open conformational change of AimR, eliminating DNA targeting. Our structural and functional analysis provides new insights into the DNA recognition mechanism of AimR and its regulation by the arbitrium peptide in the context of phage lysis-lysogeny decisions.
中文翻译:

对SPbeta噬菌体中Arimtrium通讯系统进行AimR DNA识别的结构见解
新近鉴定出的arbitrium通信系统可调节芽孢杆菌噬菌体中溶菌至溶菌的决定。该系统包含一个作为信号的Arbitrium六肽,细胞受体AimR和溶源性负调节剂AimX。AimR特异性靶向下游DNA以激活aimX基因表达。Arbitrium肽与AimR结合,抑制其DNA结合以促进噬菌体溶原性。最近,我们和其他研究小组阐明了AimR如何感知Arbitrium肽。但是,通过AimR识别DNA的分子机制及其通过肽调节其DNA结合活性的机制仍然未知。在这里,我们报告了AimR–DNA复合物在2.1Å分辨率下的晶体结构。N末端的HTH基序识别回文的DNA序列,该序列由带正电荷的残基与DNA磷酸基团之间的相互作用所支撑。与肽结合的形式相比,与DNA结合的AimR组装的二聚体更为封闭。单分子FRET和交联测定表明,AimR蛋白样品在溶液中既开放又封闭。Arbitrium肽结合诱导AimR的封闭到开放的构象变化,消除了DNA靶向。我们的结构和功能分析提供了对AimR的DNA识别机制及其在噬菌体裂解-溶原性决定背景下由Arbitrium肽进行调控的新见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号