Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A conserved PLPLRT/SD motif of STING mediates the recruitment and activation of TBK1
Nature ( IF 64.8 ) Pub Date : 2019-05-01 , DOI: 10.1038/s41586-019-1228-x Baoyu Zhao 1 , Fenglei Du 1 , Pengbiao Xu 1 , Chang Shu 1 , Banumathi Sankaran 2 , Samantha L Bell 3 , Mengmeng Liu 4 , Yuanjiu Lei 3 , Xinsheng Gao 4 , Xiaofeng Fu 5 , Fanxiu Zhu 5 , Yang Liu 6 , Arthur Laganowsky 6 , Xueyun Zheng 6 , Jun-Yuan Ji 4 , A Phillip West 3 , Robert O Watson 3 , Pingwei Li 1
Nature ( IF 64.8 ) Pub Date : 2019-05-01 , DOI: 10.1038/s41586-019-1228-x Baoyu Zhao 1 , Fenglei Du 1 , Pengbiao Xu 1 , Chang Shu 1 , Banumathi Sankaran 2 , Samantha L Bell 3 , Mengmeng Liu 4 , Yuanjiu Lei 3 , Xinsheng Gao 4 , Xiaofeng Fu 5 , Fanxiu Zhu 5 , Yang Liu 6 , Arthur Laganowsky 6 , Xueyun Zheng 6 , Jun-Yuan Ji 4 , A Phillip West 3 , Robert O Watson 3 , Pingwei Li 1
Affiliation
|
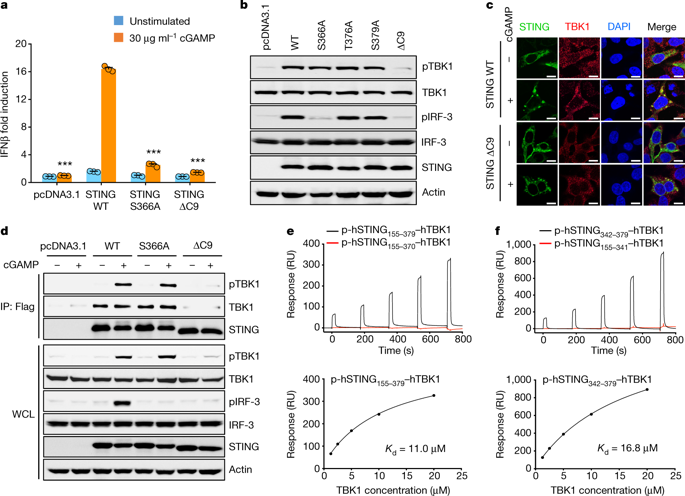
Nucleic acids from bacteria or viruses induce potent immune responses in infected cells1–4. The detection of pathogen-derived nucleic acids is a central strategy by which the host senses infection and initiates protective immune responses5,6. Cyclic GMP-AMP synthase (cGAS) is a double-stranded DNA sensor7,8. It catalyses the synthesis of cyclic GMP-AMP (cGAMP)9–12, which stimulates the induction of type I interferons through the STING–TBK1–IRF-3 signalling axis13–15. STING oligomerizes after binding of cGAMP, leading to the recruitment and activation of the TBK1 kinase8,16. The IRF-3 transcription factor is then recruited to the signalling complex and activated by TBK18,17–20. Phosphorylated IRF-3 translocates to the nucleus and initiates the expression of type I interferons21. However, the precise mechanisms that govern activation of STING by cGAMP and subsequent activation of TBK1 by STING remain unclear. Here we show that a conserved PLPLRT/SD motif within the C-terminal tail of STING mediates the recruitment and activation of TBK1. Crystal structures of TBK1 bound to STING reveal that the PLPLRT/SD motif binds to the dimer interface of TBK1. Cell-based studies confirm that the direct interaction between TBK1 and STING is essential for induction of IFNβ after cGAMP stimulation. Moreover, we show that full-length STING oligomerizes after it binds cGAMP, and highlight this as an essential step in the activation of STING-mediated signalling. These findings provide a structural basis for the development of STING agonists and antagonists for the treatment of cancer and autoimmune disorders.A molecular model of STING-mediated signalling is proposed, as structural analysis identifies a crucial motif for the binding of TBK1 to STING, and a separate model involved in IRF-3 binding.
中文翻译:

STING 的保守 PLPLRT/SD 基序介导 TBK1 的招募和激活
来自细菌或病毒的核酸在受感染的细胞中诱导有效的免疫反应1-4。病原体来源的核酸的检测是宿主感知感染并启动保护性免疫反应的核心策略5,6。环 GMP-AMP 合酶 (cGAS) 是一种双链 DNA 传感器7,8。它催化环 GMP-AMP (cGAMP)9-12 的合成,从而通过 STING-TBK1-IRF-3 信号轴刺激 I 型干扰素的诱导13-15。STING 与 cGAMP 结合后寡聚,导致 TBK1 激酶的募集和激活8,16。然后 IRF-3 转录因子被招募到信号复合物并被 TBK18,17-20 激活。磷酸化的 IRF-3 易位至细胞核并启动 I 型干扰素的表达21。然而,控制 cGAMP 激活 STING 以及随后 STING 激活 TBK1 的精确机制仍不清楚。在这里,我们表明 STING C 末端尾部内的保守 PLPLRT/SD 基序介导 TBK1 的招募和激活。TBK1 与 STING 结合的晶体结构表明 PLPLRT/SD 基序与 TBK1 的二聚体界面结合。基于细胞的研究证实,TBK1 和 STING 之间的直接相互作用对于 cGAMP 刺激后诱导 IFNβ 至关重要。此外,我们发现全长 STING 在结合 cGAMP 后发生寡聚,并强调这是激活 STING 介导的信号传导的重要步骤。这些发现为开发用于治疗癌症和自身免疫性疾病的 STING 激动剂和拮抗剂提供了结构基础。提出了 STING 介导的信号传导的分子模型,因为结构分析确定了 TBK1 与 STING 结合的关键基序,并且涉及 IRF-3 结合的单独模型。
更新日期:2019-05-01
中文翻译:

STING 的保守 PLPLRT/SD 基序介导 TBK1 的招募和激活
来自细菌或病毒的核酸在受感染的细胞中诱导有效的免疫反应1-4。病原体来源的核酸的检测是宿主感知感染并启动保护性免疫反应的核心策略5,6。环 GMP-AMP 合酶 (cGAS) 是一种双链 DNA 传感器7,8。它催化环 GMP-AMP (cGAMP)9-12 的合成,从而通过 STING-TBK1-IRF-3 信号轴刺激 I 型干扰素的诱导13-15。STING 与 cGAMP 结合后寡聚,导致 TBK1 激酶的募集和激活8,16。然后 IRF-3 转录因子被招募到信号复合物并被 TBK18,17-20 激活。磷酸化的 IRF-3 易位至细胞核并启动 I 型干扰素的表达21。然而,控制 cGAMP 激活 STING 以及随后 STING 激活 TBK1 的精确机制仍不清楚。在这里,我们表明 STING C 末端尾部内的保守 PLPLRT/SD 基序介导 TBK1 的招募和激活。TBK1 与 STING 结合的晶体结构表明 PLPLRT/SD 基序与 TBK1 的二聚体界面结合。基于细胞的研究证实,TBK1 和 STING 之间的直接相互作用对于 cGAMP 刺激后诱导 IFNβ 至关重要。此外,我们发现全长 STING 在结合 cGAMP 后发生寡聚,并强调这是激活 STING 介导的信号传导的重要步骤。这些发现为开发用于治疗癌症和自身免疫性疾病的 STING 激动剂和拮抗剂提供了结构基础。提出了 STING 介导的信号传导的分子模型,因为结构分析确定了 TBK1 与 STING 结合的关键基序,并且涉及 IRF-3 结合的单独模型。


























 京公网安备 11010802027423号
京公网安备 11010802027423号