npj Precision Oncology ( IF 7.9 ) Pub Date : 2019-04-15 , DOI: 10.1038/s41698-019-0083-4 Janire Mingo , Sandra Luna , Ayman Gaafar , Caroline E. Nunes-Xavier , Leire Torices , Lorena Mosteiro , Rebeca Ruiz , Isabel Guerra , Roberto Llarena , Javier C. Angulo , José I. López , Rafael Pulido
|
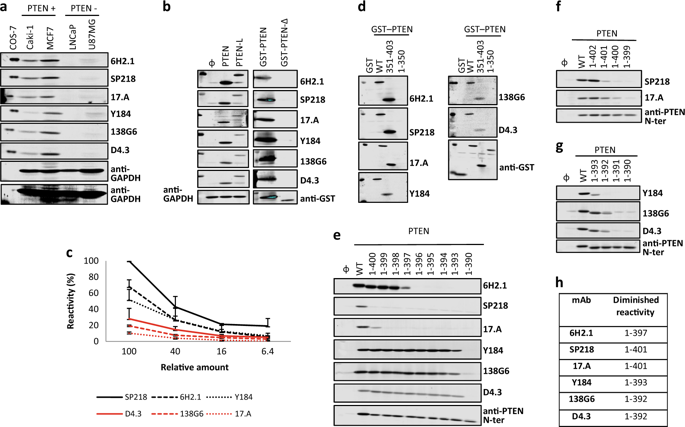
Anti-PTEN monoclonal antibodies (mAb) are arising as important tools for immunohistochemistry (IHC) and protein quantification routine analysis in clinical oncology. Although an effort has been made to document the reliability of tumor tissue section immunostaining by anti-PTEN mAb, and to standardize their IHC use in research and in the clinical practice, the precise topological and biochemical definition of the epitope recognized by each mAb has been conventionally overlooked. In this study, six commercial anti-PTEN mAb have been validated and characterized for sensitivity and specificity by IHC and FISH, using a set of prostate and urothelial bladder tumor specimens, and by immunoblot, using PTEN positive and PTEN negative human cell lines. Immunoblot precise epitope mapping, performed using recombinant PTEN variants and mutations, revealed that all mAb recognized linear epitopes of 6–11 amino acid length at the PTEN C-terminus. Tumor-associated or disease-associated mutations at the PTEN C-terminus did not affect subcellular localization or PIP3 phosphatase activity of PTEN in cells, although resulted in specific loss of reactivity for some mAb. Furthermore, specific mimicking-phosphorylation mutations at the PTEN C-terminal region also abolished binding of specific mAb. Our study adds new evidence on the relevance of a precise epitope mapping in the validation of anti-PTEN mAb for their use in the clinics. This will be substantial to provide a more accurate diagnosis in clinical oncology based on PTEN protein expression in tumors and biological fluids.
中文翻译:

PTEN C末端表位的精确定义及其在临床肿瘤学中的意义
抗PTEN单克隆抗体(mAb)逐渐成为临床肿瘤学中免疫组织化学(IHC)和蛋白质定量常规分析的重要工具。尽管已努力证明用抗PTEN mAb进行肿瘤组织切片免疫染色的可靠性,并标准化其在研究和临床实践中的IHC使用,但已经确定了每种mAb识别的表位的精确拓扑和生化定义传统上被忽略了。在这项研究中,使用一组前列腺癌和尿路上皮膀胱肿瘤标本,通过IHC和FISH验证了六种商业抗PTEN mAb的敏感性和特异性,并使用PTEN阳性和PTEN阴性人类细胞系通过免疫印迹进行了鉴定。使用重组PTEN变体和突变进行的免疫印迹精确表位作图,结果表明,所有mAb均可在PTEN C末端识别6–11个氨基酸长度的线性表位。PTEN C末端的肿瘤相关或疾病相关突变不会影响细胞中PTEN的亚细胞定位或PIP3磷酸酶活性,尽管会导致某些mAb的反应性特异性降低。此外,PTEN C末端区域的特定模仿磷酸化突变也消除了特定mAb的结合。我们的研究为精确的表位作图与抗PTEN mAb在临床上的验证相关性提供了新的证据。这对于基于肿瘤和生物体液中的PTEN蛋白表达在临床肿瘤学中提供更准确的诊断将非常重要。PTEN C末端的肿瘤相关或疾病相关突变不会影响细胞中PTEN的亚细胞定位或PIP3磷酸酶活性,尽管会导致某些mAb的反应性特异性降低。此外,PTEN C末端区域的特定模仿磷酸化突变也消除了特定mAb的结合。我们的研究为精确的表位作图与抗PTEN mAb在临床上的验证相关性提供了新的证据。这对于基于肿瘤和生物体液中的PTEN蛋白表达在临床肿瘤学中提供更准确的诊断将非常重要。PTEN C末端的肿瘤相关或疾病相关突变不会影响细胞中PTEN的亚细胞定位或PIP3磷酸酶活性,尽管会导致某些mAb的反应性特异性降低。此外,PTEN C末端区域的特定模仿磷酸化突变也消除了特定mAb的结合。我们的研究为精确的表位作图与抗PTEN mAb在临床上的验证相关性提供了新的证据。这对于基于肿瘤和生物体液中的PTEN蛋白表达在临床肿瘤学中提供更准确的诊断将非常重要。PTEN C末端区域的特定模仿磷酸化突变也消除了特定mAb的结合。我们的研究为精确的表位作图与抗PTEN mAb在临床上的验证相关性提供了新的证据。这对于基于肿瘤和生物体液中的PTEN蛋白表达在临床肿瘤学中提供更准确的诊断将非常重要。PTEN C末端区域的特定模仿磷酸化突变也消除了特定mAb的结合。我们的研究为精确的表位作图与抗PTEN mAb在临床上的验证相关性提供了新的证据。这对于基于肿瘤和生物体液中的PTEN蛋白表达在临床肿瘤学中提供更准确的诊断将非常重要。


























 京公网安备 11010802027423号
京公网安备 11010802027423号