当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Insight into G Protein-Coupled Receptor Signaling Efficacy and Bias between Gs and β-Arrestin
ACS Pharmacology & Translational Science Pub Date : 2019-04-09 , DOI: 10.1021/acsptsci.9b00012 Louis-Philippe Picard 1 , Anne-Marie Schonegge 1 , Michel Bouvier 1
ACS Pharmacology & Translational Science Pub Date : 2019-04-09 , DOI: 10.1021/acsptsci.9b00012 Louis-Philippe Picard 1 , Anne-Marie Schonegge 1 , Michel Bouvier 1
Affiliation
|
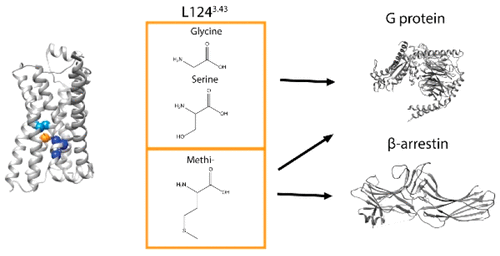
G protein-coupled receptors (GPCRs) form the largest family of membrane proteins involved in signal transduction. Because of their ability to regulate a wide range of cellular responses and their dysregulation being associated with many diseases, GPCRs remain a key therapeutic target for several clinical indications. In recent years, it has been demonstrated that ligands for a given receptor can engage distinct pathways with different relative efficacies, a concept known as biased signaling or functional selectivity. However, the structural determinants of this phenomenon remain poorly understood. Using the β2-adrenergic receptor as a model, we identified a linker residue (L1243.43) between the known PIF and NPxxY structural motifs, that plays a central role in the differential efficacy of biased ligands toward the Gs and β-arrestin pathways. Given the high level of conservation of this linker residue, the study provides structural explanations for biased signaling that can be extrapolated to other GPCRs.
中文翻译:

G蛋白偶联受体信号传导功效和Gs与β-Arrestin之间偏倚的结构洞察
G蛋白偶联受体(GPCR)构成了参与信号转导的最大膜蛋白家族。由于其调节多种细胞应答的能力以及与许多疾病相关的失调,GPCR仍然是几种临床适应症的关键治疗靶标。近年来,已经证明给定受体的配体可以以不同的相对效率参与不同的途径,这一概念被称为偏向信号或功能选择性。但是,这种现象的结构决定因素仍然知之甚少。使用β2-肾上腺素能受体作为模型,我们确定了一个接头残基(L124 3.43)在已知的PIF和NPxxY结构基序之间),在偏向Gs和β-arrestin途径的配体的差异功效中起着核心作用。鉴于该接头残基的高度保守性,该研究为可推断到其他GPCR的偏向信号提供了结构性解释。
更新日期:2019-05-23
中文翻译:

G蛋白偶联受体信号传导功效和Gs与β-Arrestin之间偏倚的结构洞察
G蛋白偶联受体(GPCR)构成了参与信号转导的最大膜蛋白家族。由于其调节多种细胞应答的能力以及与许多疾病相关的失调,GPCR仍然是几种临床适应症的关键治疗靶标。近年来,已经证明给定受体的配体可以以不同的相对效率参与不同的途径,这一概念被称为偏向信号或功能选择性。但是,这种现象的结构决定因素仍然知之甚少。使用β2-肾上腺素能受体作为模型,我们确定了一个接头残基(L124 3.43)在已知的PIF和NPxxY结构基序之间),在偏向Gs和β-arrestin途径的配体的差异功效中起着核心作用。鉴于该接头残基的高度保守性,该研究为可推断到其他GPCR的偏向信号提供了结构性解释。



























 京公网安备 11010802027423号
京公网安备 11010802027423号