Oncogenesis ( IF 6.2 ) Pub Date : 2019-04-02 , DOI: 10.1038/s41389-019-0135-1 Chun Li , Wei Xiong , Xiong Liu , Wenjun Xiao , Yuxian Guo , Junyu Tan , Yaochen Li
|
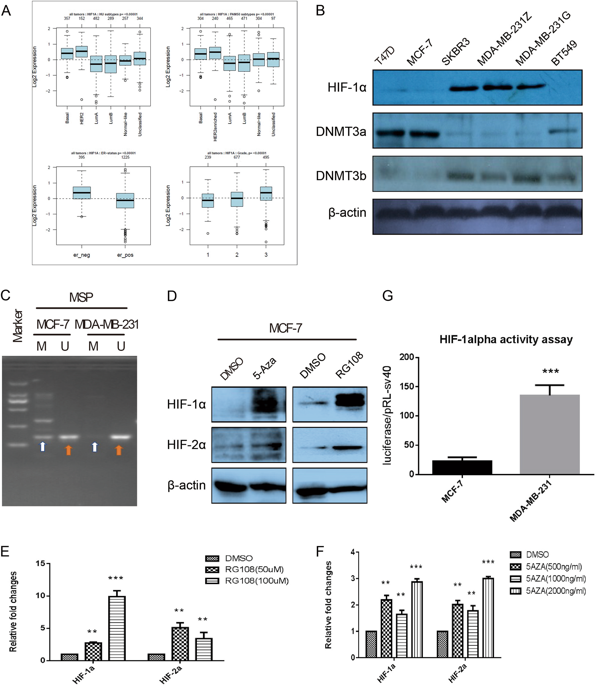
HIF-1α has a broad impact on tumors, including enhanced utilization of glucose, tumor cell stemness, migration, metastasis and so on. In pilot study, we found that the expression of HIF-1α significantly increased in breast cancer cell lines and tissue samples with higher malignant behaviors and decreased in luminal subtype breast cancer cells and tissue samples. We analyzed and found there is one large CpG island in HIF-1α promoter around transcription start site, and the hypermethylation occurred at these CpGs and their surrounding non-CpGs sites. Epigenetic events driving tumorigenesis has been characterized. However, knowledge is lacking on the non-CpGs methylation of HIF-1α promoter in breast cancer cells. We validated that non-CpGs methylation can directly regulate HIF-1α expression by luciferase activity assay. We also found DNMT3a and Mecp2 play vital role in methylation at non-CpGs and CpGs sites. In addition, we noticed that H3K9ac modification could promote the transcription of HIF-1α in MDA-MB-231 cells by binding to the region contained hypomethylated non-CpG and CpG sites. Taken together, the hypomethylation status at non-CpG and CpG loci in HIF-1α promoter and H3K9ac modification together contribute to maintain higher HIF-1αactivity in invasive breast cancer cells when compared with the non-invasive breast cancer cells, which may establish a tissue-specific epigenetic modification pattern and point to the new directions for future understanding breast cancer therapy.
中文翻译:

HIF-1α基因启动子中非CpG / CpG位点的低甲基化结合增强的H3K9Ac修饰有助于维持乳腺癌中较高的HIF-1α表达
HIF-1α对肿瘤具有广泛的影响,包括提高葡萄糖的利用,肿瘤细胞的干性,迁移,转移等。在初步研究中,我们发现HIF-1α的表达在具有较高恶性行为的乳腺癌细胞系和组织样品中显着增加,而在管腔亚型乳腺癌细胞和组织样品中降低。我们分析发现在转录起始位点附近的HIF-1α启动子中有一个大的CpG岛,并且高甲基化发生在这些CpGs及其周围的非CpGs位点。已经表征了驱动肿瘤发生的表观遗传事件。然而,缺乏关于乳腺癌细胞中HIF-1α启动子的非CpGs甲基化的知识。我们通过萤光素酶活性试验验证了非CpGs甲基化可以直接调节HIF-1α的表达。我们还发现DNMT3a和Mecp2在非CpGs和CpGs位点的甲基化中起着至关重要的作用。此外,我们注意到H3K9ac修饰可以通过与包含低甲基化的非CpG和CpG位点的区域结合来促进MDA-MB-231细胞中HIF-1α的转录。两者合计,与非侵入性乳腺癌细胞相比,HIF-1α启动子和H3K9ac修饰的非CpG和CpG基因座处的低甲基化状态共同有助于维持较高的HIF-1α活性,这可能会建立组织特定的表观遗传修饰模式,并为今后了解乳腺癌治疗方法指明了新的方向。我们注意到,H3K9ac修饰可以通过与包含低甲基化的非CpG和CpG位点的区域结合来促进MDA-MB-231细胞中HIF-1α的转录。两者合计,与非侵入性乳腺癌细胞相比,HIF-1α启动子和H3K9ac修饰的非CpG和CpG基因座处的低甲基化状态共同有助于维持较高的HIF-1α活性,这可能会建立组织特定的表观遗传修饰模式,并为今后了解乳腺癌治疗方法指明了新的方向。我们注意到,H3K9ac修饰可以通过与包含低甲基化的非CpG和CpG位点的区域结合来促进MDA-MB-231细胞中HIF-1α的转录。两者合计,与非侵入性乳腺癌细胞相比,HIF-1α启动子和H3K9ac修饰的非CpG和CpG基因座处的低甲基化状态共同有助于维持较高的HIF-1α活性,这可能会建立组织特定的表观遗传修饰模式,并为今后了解乳腺癌治疗方法指明了新的方向。



























 京公网安备 11010802027423号
京公网安备 11010802027423号