当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identification of Key Structural Motifs Involved in 7 Transmembrane Signaling of Adhesion GPCRs
ACS Pharmacology & Translational Science Pub Date : 2019-02-15 , DOI: 10.1021/acsptsci.8b00051 Marta Arimont 1 , Melanie van der Woude 1 , Rob Leurs 1 , Henry F. Vischer 1 , Chris de Graaf 1 , Saskia Nijmeijer 1
ACS Pharmacology & Translational Science Pub Date : 2019-02-15 , DOI: 10.1021/acsptsci.8b00051 Marta Arimont 1 , Melanie van der Woude 1 , Rob Leurs 1 , Henry F. Vischer 1 , Chris de Graaf 1 , Saskia Nijmeijer 1
Affiliation
|
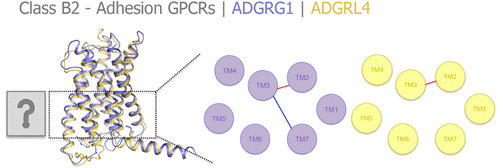
The adhesion class B2 family of G protein-coupled receptors (GPCRs) plays a key role in important physiological processes and their dysfunction is linked to brain malformations and tumorigenesis. Although information regarding their signaling properties is starting to emerge, the structural motifs and interaction networks that determine 7 transmembrane (TM) signaling of class B2 GPCRs remain to be elucidated. Comparative sequence–structure analyses of class B2 GPCRs and the recently solved active class B1 structures show that class B2 GPCRs include different elements of the conserved residue motifs that determine class B1 activation. Combined site-directed mutagenesis and molecular dynamics studies were performed to give detailed insights into the role of 7TM interaction networks in signaling of two representative class B2 receptors, ADGRG1 (GPR56) and ADGRL4 (ELTD1). The systematic investigation of class B1/B2 sequence motifs provides consistent structure–function relationships that can be translated to the whole class B2 GPCR family and suggests that class B1 and B2 GPCRs share conserved intramolecular 7TM interactions. This improved understanding in adhesion GPCR structure and constitutive signaling can accelerate drug design campaigns for this chemically unexplored receptor class.
中文翻译:

鉴定参与粘附GPCR的7个跨膜信号的关键结构基元。
G蛋白偶联受体(GPCR)的粘附性B2家族在重要的生理过程中起关键作用,其功能障碍与脑畸形和肿瘤发生有关。尽管有关其信号传导特性的信息开始出现,但确定B2类GPCR的7个跨膜(TM)信号传导的结构基序和相互作用网络仍有待阐明。对B2类GPCR和最近解决的活性B1类结构的比较序列结构分析表明,B2 GPCR类包括确定B1类激活的保守残基基序的不同元素。进行了联合定点诱变和分子动力学研究,以深入了解7TM相互作用网络在两个代表性B2类受体信号传导中的作用,ADGRG1(GPR56)和ADGRL4(ELTD1)。对B1 / B2类序列基序的系统研究提供了一致的结构-功能关系,可以将其翻译为整个B2 GPCR类,并表明B1和B2 GPCR类具有保守的分子内7TM相互作用。对粘附GPCR结构和组成型信号传导的这种更好的理解可以加速针对这种化学未探索的受体类别的药物设计运动。
更新日期:2019-04-01
中文翻译:

鉴定参与粘附GPCR的7个跨膜信号的关键结构基元。
G蛋白偶联受体(GPCR)的粘附性B2家族在重要的生理过程中起关键作用,其功能障碍与脑畸形和肿瘤发生有关。尽管有关其信号传导特性的信息开始出现,但确定B2类GPCR的7个跨膜(TM)信号传导的结构基序和相互作用网络仍有待阐明。对B2类GPCR和最近解决的活性B1类结构的比较序列结构分析表明,B2 GPCR类包括确定B1类激活的保守残基基序的不同元素。进行了联合定点诱变和分子动力学研究,以深入了解7TM相互作用网络在两个代表性B2类受体信号传导中的作用,ADGRG1(GPR56)和ADGRL4(ELTD1)。对B1 / B2类序列基序的系统研究提供了一致的结构-功能关系,可以将其翻译为整个B2 GPCR类,并表明B1和B2 GPCR类具有保守的分子内7TM相互作用。对粘附GPCR结构和组成型信号传导的这种更好的理解可以加速针对这种化学未探索的受体类别的药物设计运动。

























 京公网安备 11010802027423号
京公网安备 11010802027423号