当前位置:
X-MOL 学术
›
Blood Cancer J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phase I trial of isatuximab monotherapy in the treatment of refractory multiple myeloma.
Blood Cancer Journal ( IF 12.8 ) Pub Date : 2019-03-29 , DOI: 10.1038/s41408-019-0198-4 Thomas Martin 1 , Stephen Strickland 2 , Martha Glenn 3 , Eric Charpentier 4 , Hélène Guillemin 5 , Karl Hsu 4 , Joseph Mikhael 6
Blood Cancer Journal ( IF 12.8 ) Pub Date : 2019-03-29 , DOI: 10.1038/s41408-019-0198-4 Thomas Martin 1 , Stephen Strickland 2 , Martha Glenn 3 , Eric Charpentier 4 , Hélène Guillemin 5 , Karl Hsu 4 , Joseph Mikhael 6
Affiliation
|
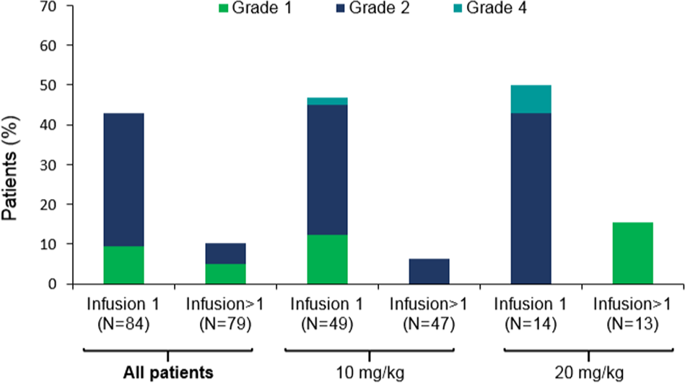
This phase I dose-escalation/expansion study evaluated isatuximab (anti-CD38 monoclonal antibody) monotherapy in patients with relapsed/refractory multiple myeloma (RRMM). Patients progressing on or after standard therapy received intravenous isatuximab (weekly [QW] or every 2 weeks [Q2W]). The primary objective was to determine the maximum tolerated dose (MTD) of isatuximab. Overall, 84 patients received ≥ 1 dose of isatuximab. The MTD was not reached; no cumulative adverse reactions were noted. The most frequent adverse events were infusion reactions (IRs), occurring in 37/73 patients (51%) following introduction of mandatory prophylaxis. IRs were mostly grade 1/2, occurred predominantly during Cycle 1, and led to treatment discontinuation in two patients. CD38 receptor occupancy reached a plateau of 80% with isatuximab 20 mg/kg (highest dose tested) and was associated with clinical response. In patients receiving isatuximab ≥ 10 mg/kg, overall response rate (ORR) was 23.8% (15/63), including one complete response. In high-risk patients treated with isatuximab 10 mg/kg (QW or Q2W), ORR was 16.7% (3/18). Median (range) duration of response at doses ≥ 10 mg/kg was 25 (8-30) weeks among high-risk patients versus 36 (6-85) weeks for other patients. In conclusion, isatuximab demonstrated a manageable safety profile and clinical activity in patients with RRMM.
中文翻译:

艾沙妥昔单抗单药治疗难治性多发性骨髓瘤的一期临床试验。
这项I期剂量递增/扩展研究评估了复发/难治性多发性骨髓瘤(RRMM)患者的isatuximab(抗CD38单克隆抗体)单药治疗。在标准治疗中或治疗后进展的患者接受静脉注射艾妥昔单抗(每周[QW]或每2周[Q2W])。主要目的是确定艾沙妥昔单抗的最大耐受剂量(MTD)。总体而言,有84例患者接受了≥1剂量的伊妥昔单抗治疗。未达到MTD;没有发现累积的不良反应。引入强制性预防措施后,最常见的不良事件是输注反应(IR),在37/73例患者中发生(51%)。IR大多为1/2级,主要发生在第1周期,导致两名患者中止治疗。异烟单抗20 mg / kg(测试的最高剂量)的CD38受体占有率达到80%的平稳期,并与临床反应相关。接受伊妥昔单抗≥10 mg / kg的患者,总缓解率(ORR)为23.8%(15/63),包括一项完全缓解。在接受10 mg / kg isatuximab(QW或Q2W)治疗的高危患者中,ORR为16.7%(3/18)。在高危患者中,≥10 mg / kg剂量的反应中位(持续时间)为25(8-30)周,而其他患者为36(6-85)周。总之,伊沙妥昔单抗在RRMM患者中显示出可控的安全性和临床活动。ORR为16.7%(3/18)。在高危患者中,≥10 mg / kg剂量的反应中位(持续时间)为25(8-30)周,而其他患者为36(6-85)周。总之,伊沙妥昔单抗在RRMM患者中显示出可控的安全性和临床活动。ORR为16.7%(3/18)。在高危患者中,≥10 mg / kg剂量的反应中位(持续时间)为25(8-30)周,而其他患者为36(6-85)周。总之,伊沙妥昔单抗在RRMM患者中显示出可控的安全性和临床活动。
更新日期:2019-11-18
中文翻译:

艾沙妥昔单抗单药治疗难治性多发性骨髓瘤的一期临床试验。
这项I期剂量递增/扩展研究评估了复发/难治性多发性骨髓瘤(RRMM)患者的isatuximab(抗CD38单克隆抗体)单药治疗。在标准治疗中或治疗后进展的患者接受静脉注射艾妥昔单抗(每周[QW]或每2周[Q2W])。主要目的是确定艾沙妥昔单抗的最大耐受剂量(MTD)。总体而言,有84例患者接受了≥1剂量的伊妥昔单抗治疗。未达到MTD;没有发现累积的不良反应。引入强制性预防措施后,最常见的不良事件是输注反应(IR),在37/73例患者中发生(51%)。IR大多为1/2级,主要发生在第1周期,导致两名患者中止治疗。异烟单抗20 mg / kg(测试的最高剂量)的CD38受体占有率达到80%的平稳期,并与临床反应相关。接受伊妥昔单抗≥10 mg / kg的患者,总缓解率(ORR)为23.8%(15/63),包括一项完全缓解。在接受10 mg / kg isatuximab(QW或Q2W)治疗的高危患者中,ORR为16.7%(3/18)。在高危患者中,≥10 mg / kg剂量的反应中位(持续时间)为25(8-30)周,而其他患者为36(6-85)周。总之,伊沙妥昔单抗在RRMM患者中显示出可控的安全性和临床活动。ORR为16.7%(3/18)。在高危患者中,≥10 mg / kg剂量的反应中位(持续时间)为25(8-30)周,而其他患者为36(6-85)周。总之,伊沙妥昔单抗在RRMM患者中显示出可控的安全性和临床活动。ORR为16.7%(3/18)。在高危患者中,≥10 mg / kg剂量的反应中位(持续时间)为25(8-30)周,而其他患者为36(6-85)周。总之,伊沙妥昔单抗在RRMM患者中显示出可控的安全性和临床活动。


























 京公网安备 11010802027423号
京公网安备 11010802027423号