npj Aging and Mechanisms of Disease Pub Date : 2018-02-13 , DOI: 10.1038/s41514-018-0021-7 Hironori Nakagami , Ken Sugimoto , Takahiro Ishikawa , Taku Fujimoto , Toshifumi Yamaoka , Misa Hayashi , Eiji Kiyohara , Hiroshi Ando , Yuta Terabe , Yoichi Takami , Koichi Yamamoto , Yasushi Takeya , Minoru Takemoto , Masaya Koshizaka , Tamotsu Ebihara , Ayumi Nakamura , Mitsunori Nishikawa , Xiang Jing Yao , Hideki Hanaoka , Ichiro Katayama , Koutaro Yokote , Hiromi Rakugi
|
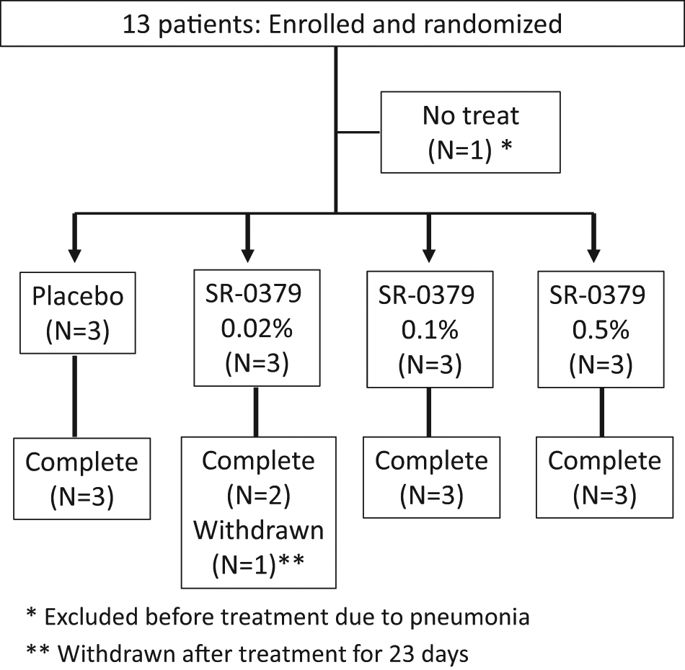
SR-0379 is a functional peptide that has wound healing effect with anti-microbial action, making it an ideal drug to prevent infection. To evaluate the safety, efficacy, and pharmacokinetics of SR-0379 for the treatment of leg ulcers, a physician-initiated, phase I/IIa, first-in-patient clinical study was designed. A multi-center, double-blind, randomized clinical study was conducted from October 2015 to September 2016. The inclusion criteria for leg ulcers were (1) diabetes or critical limb ischemia and (2) wound size <6 cm in diameter. Twelve patients were randomized into four groups and administered 0.02%, 0.1%, or 0.5% SR-0379 or placebo treatment on skin ulcers once per day for 28 days. Efficiency was evaluated by determining the rate of wound size reduction as a primary endpoint at 4 weeks after the first treatment compared with the pre-treatment wound size. As a secondary endpoint, the DESIGN-R score index, time to wound closure, and the 50% wound size reduction ratio were also evaluated. The safety of SR-0379 was evaluated during the study period. In the evaluation of efficiency, the skin ulcer reduction rates at the last evaluation were 44.73% for the 0.02% SR-0379 group, 68.25% for the 0.1% group, and 71.61% for the 0.5% group, compared with 9.95% for the placebo group. Six adverse events were reported in four patients, of which one occurred in the placebo group, and causal relationships to study drugs were denied for all six events. Treatment with SR-0379 for chronic leg ulcers was safe, well tolerated, and effective.
中文翻译:

由医师启动的用功能肽SR-0379治疗的肢体溃疡的临床研究:从发现到随机,双盲,安慰剂对照试验
SR-0379是一种功能肽,具有伤口愈合作用和抗微生物作用,使其成为预防感染的理想药物。为了评估SR-0379在治疗腿部溃疡中的安全性,疗效和药代动力学,设计了一项由医生启动的I / IIa期,首次住院患者的临床研究。从2015年10月至2016年9月,进行了一项多中心,双盲,随机临床研究。腿部溃疡的纳入标准为(1)糖尿病或严重肢体缺血和(2)直径小于6 cm的伤口。将十二名患者随机分为四组,每天一次对皮肤溃疡进行0.02%,0.1%或0.5%SR-0379或安慰剂治疗,持续28天。通过确定与治疗前伤口大小相比,首次治疗后4周的伤口大小减少率作为主要终点,来评估效率。作为次要终点,还评估了DESIGN-R得分指数,伤口闭合时间和50%的伤口缩小率。在研究期间评估了SR-0379的安全性。在效率评估中,上一次评估的皮肤溃疡减少率在0.02%SR-0379组中为44.73%,在0.1%组中为68.25%,在0.5%组中为71.61%,而上次评估为9.95%。安慰剂组。在四名患者中报告了六种不良事件,其中一例在安慰剂组中发生,并且所有六项事件均与研究药物的因果关系被否认。SR-0379治疗慢性腿部溃疡是安全,耐受性良好且有效的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号