Immunity ( IF 32.4 ) Pub Date : 2019-03-19 , DOI: 10.1016/j.immuni.2019.02.016 Yunyun Yang , Liping Li , Linjie Yuan , Xiaoying Zhou , Jianxin Duan , Hongying Xiao , Ningning Cai , Shuai Han , Xianqiang Ma , Weidong Liu , Chun-Chi Chen , Lingle Wang , Xin Li , Jiahuan Chen , Ning Kang , Jing Chen , Zhixun Shen , Satish R. Malwal , Wanli Liu , Yan Shi , Eric Oldfield , Rey-Ting Guo , Yonghui Zhang
|
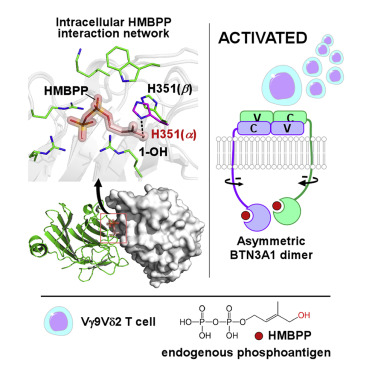
Human Vγ9Vδ2 T cells respond to microbial infections and malignancy by sensing diphosphate-containing metabolites called phosphoantigens, which bind to the intracellular domain of butyrophilin 3A1, triggering extracellular interactions with the Vγ9Vδ2 T cell receptor (TCR). Here, we examined the molecular basis of this “inside-out” triggering mechanism. Crystal structures of intracellular butyrophilin 3A proteins alone or in complex with the potent microbial phosphoantigen HMBPP or a synthetic analog revealed key features of phosphoantigens and butyrophilins required for γδ T cell activation. Analyses with chemical probes and molecular dynamic simulations demonstrated that dimerized intracellular proteins cooperate in sensing HMBPP to enhance the efficiency of γδ T cell activation. HMBPP binding to butyrophilin doubled the binding force between a γδ T cell and a target cell during “outside” signaling, as measured by single-cell force microscopy. Our findings provide insight into the “inside-out” triggering of Vγ9Vδ2 T cell activation by phosphoantigen-bound butyrophilin, facilitating immunotherapeutic drug design.
中文翻译:

磷酸抗原结合后酪氨酸蛋白的结构变化是磷酸抗原介导的Vγ9Vδ2T细胞活化的基础。
人类的Vγ9Vδ2T细胞通过感知称为磷酸抗原的含二磷酸盐的代谢产物对微生物感染和恶性肿瘤做出反应,这些代谢产物与嗜丁绿素3A1的胞内域结合,触发与Vγ9Vδ2T细胞受体(TCR)的细胞外相互作用。在这里,我们研究了这种“由内而外”触发机制的分子基础。单独或与有效的微生物磷酸抗原HMBPP或合成的类似物复合的细胞内嗜酪蛋白3A蛋白的晶体结构揭示了γδT细胞活化所需的磷酸抗原和嗜丁霉素的关键特征。用化学探针和分子动力学模拟进行的分析表明,二聚化的细胞内蛋白可协同传感HMBPP,以增强γδT细胞活化的效率。通过单细胞力显微镜观察,在“外部”信号传递过程中,HMBPP与嗜丁菌素的结合使γδT细胞与靶细胞之间的结合力增加了一倍。我们的发现提供了由磷酸化抗原结合的丁酰荧光素“由内而外”触发Vγ9Vδ2T细胞活化的见解,从而促进了免疫治疗药物的设计。


























 京公网安备 11010802027423号
京公网安备 11010802027423号