当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ionization‐Facilitated Formation of 2D (Alumino)Silicate–Noble Gas Clathrate Compounds
Advanced Functional Materials ( IF 19.0 ) Pub Date : 2019-03-06 , DOI: 10.1002/adfm.201806583 Jian‐Qiang Zhong 1 , Mengen Wang 1, 2 , Nusnin Akter 1, 2 , John D. Kestell 1 , Tianchao Niu 1 , Alejandro M. Boscoboinik 3, 4 , Taejin Kim 2 , Dario J. Stacchiola 1 , Qin Wu 1 , Deyu Lu 1 , Jorge Anibal Boscoboinik 1
Advanced Functional Materials ( IF 19.0 ) Pub Date : 2019-03-06 , DOI: 10.1002/adfm.201806583 Jian‐Qiang Zhong 1 , Mengen Wang 1, 2 , Nusnin Akter 1, 2 , John D. Kestell 1 , Tianchao Niu 1 , Alejandro M. Boscoboinik 3, 4 , Taejin Kim 2 , Dario J. Stacchiola 1 , Qin Wu 1 , Deyu Lu 1 , Jorge Anibal Boscoboinik 1
Affiliation
|
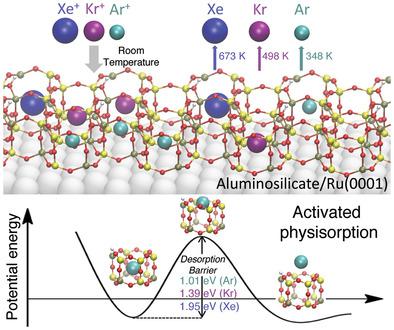
The nanoscale confinement of noble gases at noncryogenic temperatures is crucial for many applications including noble gas separations, nuclear waste remediation, and the removal of radon. However, this process is extremely difficult primarily due to the weak trapping forces of the host matrices upon noble gas physisorption. Herein, the formation of 2D clathrate compounds, which result from trapping noble gas atoms (Ar, Kr, and Xe) inside nanocages of ultrathin silica and aluminosilicate crystalline nanoporous frameworks at 300 K, is reported. The formation of the 2D clathrate compounds is attributed to a novel activated physisorption mechanism, facilitated by ionization of noble gas atoms. Combined X‐ray photoelectron spectroscopy (XPS) and density functional theory (DFT) studies provide evidence of an initial ionization process that significantly reduces the apparent trapping barrier. Noble gas ions become neutralized upon entering the cages, and their desorption requires unprecedentedly high temperatures, even in ultrahigh vacuum conditions. From 2D aluminosilicate films these temperatures are 348 K (Ar), 498 K (Kr), and 673 K (Xe). DFT calculations also predict that Rn can be trapped in 2D aluminosilicates with an even higher desorption temperature of 775 K. This work highlights a new ionization‐facilitated trapping mechanism resulting in the thinnest family of clathrates ever reported.
中文翻译:

电离促进形成2D(铝)硅酸盐-稀有气体包合物
在非低温条件下,稀有气体的纳米级封闭对于许多应用至关重要,包括稀有气体分离,核废料修复和ra的去除。但是,该过程极其困难,主要是由于主体基质在稀有气体物理吸附时的捕获力弱。在此,据报道形成了二维笼形化合物,这是由于在300 K的超薄二氧化硅和硅铝酸盐晶体纳米多孔骨架的纳米笼中捕获了稀有气体原子(Ar,Kr和Xe)导致的。2D笼形化合物的形成归因于惰性气体原子的电离促进了一种新型活化的物理吸附机制。X射线光电子能谱(XPS)和密度泛函理论(DFT)的结合研究提供了初始电离过程的证据,该过程显着降低了表观俘获势垒。惰性气体离子进入笼子后被中和,其解吸需要前所未有的高温,即使在超高真空条件下也是如此。在二维硅铝酸盐薄膜中,这些温度分别为348 K(Ar),498 K(Kr)和673 K(Xe)。DFT计算还预测,Rn可以被更高的775 K解吸温度捕获在2D硅铝酸盐中。这项工作强调了一种新的电离促进捕集机制,导致有史以来最薄的笼形螯合物家族。它们的解吸需要前所未有的高温,即使在超高真空条件下也是如此。在二维硅铝酸盐薄膜中,这些温度分别为348 K(Ar),498 K(Kr)和673 K(Xe)。DFT计算还预测,Rn可以被更高的775 K解吸温度捕获在2D硅铝酸盐中。这项工作强调了一种新的电离促进捕集机制,导致有史以来最薄的笼形螯合物家族。它们的解吸需要前所未有的高温,即使在超高真空条件下也是如此。对于二维硅铝酸盐薄膜,这些温度分别为348 K(Ar),498 K(Kr)和673 K(Xe)。DFT计算还预测,Rn可以被更高的775 K解吸温度捕获在2D硅铝酸盐中。这项工作着重说明了一种新的电离促进捕集机制,导致了迄今为止报道的最薄的笼形螯合物家族。
更新日期:2019-03-06
中文翻译:

电离促进形成2D(铝)硅酸盐-稀有气体包合物
在非低温条件下,稀有气体的纳米级封闭对于许多应用至关重要,包括稀有气体分离,核废料修复和ra的去除。但是,该过程极其困难,主要是由于主体基质在稀有气体物理吸附时的捕获力弱。在此,据报道形成了二维笼形化合物,这是由于在300 K的超薄二氧化硅和硅铝酸盐晶体纳米多孔骨架的纳米笼中捕获了稀有气体原子(Ar,Kr和Xe)导致的。2D笼形化合物的形成归因于惰性气体原子的电离促进了一种新型活化的物理吸附机制。X射线光电子能谱(XPS)和密度泛函理论(DFT)的结合研究提供了初始电离过程的证据,该过程显着降低了表观俘获势垒。惰性气体离子进入笼子后被中和,其解吸需要前所未有的高温,即使在超高真空条件下也是如此。在二维硅铝酸盐薄膜中,这些温度分别为348 K(Ar),498 K(Kr)和673 K(Xe)。DFT计算还预测,Rn可以被更高的775 K解吸温度捕获在2D硅铝酸盐中。这项工作强调了一种新的电离促进捕集机制,导致有史以来最薄的笼形螯合物家族。它们的解吸需要前所未有的高温,即使在超高真空条件下也是如此。在二维硅铝酸盐薄膜中,这些温度分别为348 K(Ar),498 K(Kr)和673 K(Xe)。DFT计算还预测,Rn可以被更高的775 K解吸温度捕获在2D硅铝酸盐中。这项工作强调了一种新的电离促进捕集机制,导致有史以来最薄的笼形螯合物家族。它们的解吸需要前所未有的高温,即使在超高真空条件下也是如此。对于二维硅铝酸盐薄膜,这些温度分别为348 K(Ar),498 K(Kr)和673 K(Xe)。DFT计算还预测,Rn可以被更高的775 K解吸温度捕获在2D硅铝酸盐中。这项工作着重说明了一种新的电离促进捕集机制,导致了迄今为止报道的最薄的笼形螯合物家族。


























 京公网安备 11010802027423号
京公网安备 11010802027423号