Journal of Molecular Catalysis A: Chemical Pub Date : 2016-08-31 , DOI: 10.1016/j.molcata.2016.08.033 A. Simaioforidou , M. Papastergiou , A. Margellou , D. Petrakis , M. Louloudi
|
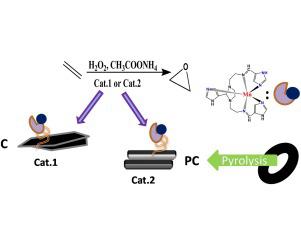
Two types of heterogeneous catalytic materials, MnII-L3imid@Cox and MnII-L3imid@PCox, have been synthesized and compared by covalent grafting of a catalytically active [MnII-L3imid] complex on the surface of an oxidized activated carbon (Cox) and an oxidized pyrolytic carbon from recycled-tire char (PCox). Both hybrids are non-porous bearing graphitic layers intermixed with disordered sp2/sp3 carbon units. Raman spectra show that (ID/IG)activatedcarbon > (ID/IG)pyrolyticcarbon revealing that oxidized activated carbon(Cox) is less graphitized than oxidized pyrolytic carbon (PCox). The MnII-L3imid@Cox and MnII-L3imid@PCox catalysts were evaluated for alkene oxidation with H2O2 in the presence of CH3COONH4. Both showed high selectivity towards epoxides and comparing the achieved yields and TONs, they appear equivalent. However, MnII-L3imid@PCox catalyst is kinetically faster than the MnII-L3imid@Cox (accomplishing the catalytic runs in 1.5 h vs. 5 h). Thus, despite the similarity in TONs MnII-L3imid@PCox achieved extremely higher TOFs vs. MnII-L3imid@Cox. Intriguingly, in terms of recyclability, MnII-L3imid@Cox could be reused for a 2th run showing a ∼20% loss of its catalytic activity, while MnII-L3imid@PCox practically no recyclable. This phenomenon is discussed in a mechanistic context; interlinking oxidative destruction of the Mn-complex with high TOFs for MnII-L3imid@PCox, while the low-TOFs of MnII-L3imid@Cox are preventive for the oxidative destruction of the Mn-complex.
中文翻译:

活化碳与热解碳作为化学功能化的载体基质:用于H 2 O 2烯烃氧化的高效多相非血红素Mn(II)催化剂
合成了两种类型的非均相催化材料,即Mn II -L 3 imid @ Cox和Mn II -L 3 imid @ PCox,并通过共价接枝了具有催化活性的[ Mn II -L 3 imid]络合物进行了比较。废轮胎焦(PCox)中的氧化活性炭(Cox)和热解炭氧化而成。两种杂化物都是无孔的石墨层,混合有无序的sp 2 / sp 3碳单元。拉曼光谱表明(I D / I G)activatedcarbon >(I d / I G ^)pyrolyticcarbon揭示氧化活性炭(COX)比氧化热解碳(低石墨化PCox) 。的锰II -L 3 IMID @考克斯和锰II -L 3 IMID @ PCox催化剂用H烯烃氧化进行了评价2 ö 2在CH的存在3 COONH 4。两者均显示出对环氧化物的高选择性,并且比较了所获得的产率和TON,它们看起来是等效的。但是,Mn II -L3 imid @ PCox催化剂在动力学上比Mn II -L 3 imid @ Cox催化剂快(完成了1.5小时vs. 5小时的催化运行)。因此,尽管在吨的相似性的Mn II -L 3 IMID @ PCox实现极其高的Tofs VS。Mn II -L 3 imid @ Cox。有趣的是,就可回收性而言, Mn II -L 3 imid @ Cox可重复使用第二次,显示其催化活性损失约20%,而Mn II -L 3 imid @ PCox几乎没有可回收资源。在机械方面讨论了这种现象。Mn II -L 3 imid @ PCox具有高TOF的Mn络合物的相互氧化破坏,而Mn II -L 3 imid @ Cox的低TOF则可以防止Mn复合物的氧化破坏。


























 京公网安备 11010802027423号
京公网安备 11010802027423号