Journal of Molecular Catalysis A: Chemical Pub Date : 2016-06-28 , DOI: 10.1016/j.molcata.2016.06.028 Marina V. Kirillova , Carla I.M. Santos , Wenyu Wu , Yu Tang , Alexander M. Kirillov
|
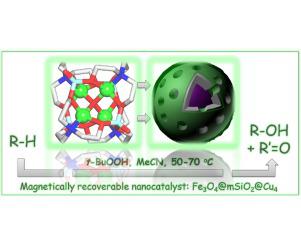
A new hybrid Fe3O4@mSiO2@Cu4 material was constructed by loading a bio-inspired tetracopper(II) coordination compound [Cu4(μ4-O){N(CH2CH2O)3}4(BOH)4][BF4]2 (Cu4) onto the Fe3O4@mSiO2 core-shell nanoparticles (NPs) composed of a magnetite (Fe3O4) core and mesoporous silica (mSiO2) shell with perpendicularly aligned channels. The obtained Fe3O4@mSiO2@Cu4 magnetic nanoparticles were characterized by transmission electron microscopy (TEM), FT-IR spectroscopy, thermogravimetric analysis (TGA), powder X-ray diffraction (PXRD), and field-dependent magnetization. This hybrid material acts as a magnetically recoverable C−H functionalization nanocatalyst, namely for the mild oxidation, by t-butyl hydroperoxide at 50–70 °C in acetonitrile medium, of cycloalkanes (cyclopentane, cyclohexane, cycloheptane, and cyclooctane) to the corresponding alcohols and ketones (with up to ∼15% yields based on cycloalkane; TON 335). A related oxidation process using cyclohexanol as a more reactive substrate leads to the formation of cyclohexanone in up to ∼25% yield (TON 570). The Fe3O4@mSiO2@Cu4 nanocatalyst can be recycled five times without an appreciable loss of activity. The bond-, regio-, and stereoselectivity parameters were investigated in the oxidation of different alkane substrates (n-hexane, n-heptane, n-octane, methylcyclohexane, adamantane, cis- and trans-1,2-demethylcyclohexane), and the obtained results were compared with the homogeneous systems based on the Cu4 catalyst. In particular, the high bond selectivity parameters detected in the oxidation of methylcyclohexane (1°:2°:3° of 1:8:142) and adamantane (2°:3° of 1:21) catalyzed by Fe3O4@mSiO2@Cu4 suggest that the reactions possibly occur in hydrophobic pockets of the nanocatalyst.
中文翻译:

使用磁性核-壳Fe 3 O 4 @mSiO 2 @Cu 4纳米催化剂对烷烃和醇类进行轻度氧化CH-H官能化
一种新的混合的Fe 3 ö 4 @mSiO 2 @Cu 4材料通过加载仿生tetracopper(II)配位化合物[铜构成4(μ 4 -O){N(CH 2 CH 2 O)3 } 4( BOH)4 ] [BF 4 ] 2(Cu 4)置于由磁铁矿(Fe 3 O 4)核和介孔二氧化硅(mSiO 2)组成的Fe 3 O 4 @mSiO 2核-壳纳米颗粒(NPs)上)外壳,且通道垂直对齐。通过透射电子显微镜(TEM),傅立叶变换红外光谱,热重分析(TGA),粉末X射线衍射(PXRD)和场相关磁化对获得的Fe 3 O 4 @mSiO 2 @Cu 4磁性纳米粒子进行表征。这种杂化材料充当磁性可回收的CH官能化纳米催化剂,即通过t的温和氧化在50-70°C的乙腈介质中,将环烷烃(环戊烷,环己烷,环庚烷和环辛烷)转化为相应的醇和酮(基于环烷烃的收率最高可达15%; TON 335)。使用环己醇作为更具反应性的底物的相关氧化过程导致形成环己酮的产率最高约为25%(TON 570)。Fe 3 O 4 @mSiO 2 @Cu 4纳米催化剂可以循环使用五次,而活性没有明显下降。该成键选择性,区域选择性,立体选择性和参数在不同烷烃底物的氧化进行了研究(Ñ己烷,Ñ -庚烷,Ñ辛烷,甲基环己烷,金刚烷,顺式和反式-1,2-脱甲基环己烷),并将所得结果与基于Cu 4催化剂的均相体系进行比较。(:2°:3°的1:8:142 1°)和金刚烷特别地,高结合选择参数在甲基环己烷中的氧化检测(2°:1:21 3°)的Fe催化的3 ö 4 @ mSiO 2 @Cu 4提示反应可能发生在纳米催化剂的疏水腔中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号