Catalysis Today ( IF 5.3 ) Pub Date : 2019-02-13 , DOI: 10.1016/j.cattod.2019.02.021 Md Ashraful Abedin , Swarom Kanitkar , Srikar Bhattar , James J. Spivey
|
One of the most challenging aspects of modern day catalysis is the conversion of methane. Natural gas is an abundant source of supply of methane and is currently being used mostly for electricity generation, and much of it is simply flared. The potential to convert methane into higher-value hydrocarbons is significant. Among other reactions, direct conversion of methane via dehydroaromatization (MDHA) can be used to produce hydrogen and valuable hydrocarbons like benzene.
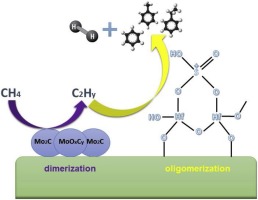
Mo oxide supported on ZSM-5/MCM-22 has been studied extensively in recent years for MDHA. It has been reported that Mo carbides are responsible for activating methane by forming CHx species. These are dimerized into C2Hy and then oligomerized on the strong ZSM-5/MCM-22 Brønsted acid sites to form benzene. Related work has shown that sulfated zirconia (SZ) provides the acid sites for Mo needed to produce benzene in MDHA [1]. The similarity of sulfated hafnia (SH) with sulfated zirconia is a logical novel support for MDHA. Although SH has been used for other acid-catalyzed reactions, we are aware of no systematic study of Mo-SH for the MDHA reaction, despite the appeal of such a catalyst. Here, Mo/SH catalysts have been tested and characterized using SEM-EDS, XPS, XANES, DRIFTS, HR-TEM, BET and temperature programmed techniques. DRIFTS confirms that SH acidity is unaffected by the addition of Mo to the catalyst. SEM-EDS and XPS have been used to characterize the loading of Mo and sulfur on SH support. XANES as well as HR-TEM analysis are used to study the formation of molybdenum carbide/ oxycarbide species, which are generally regarded to be the active sites for MDHA. MDHA runs were carried out to study the effect of Mo loading, temperature and space velocity. 5% Mo-SH is catalyst more active than 1% Mo-SH, as measured by methane conversion. Conversion increased with higher temperature and lower space velocity and gradually deactivated with time. This can be attributed to catalytic surface coking, confirmed with subsequent TPO analysis. Products observed were primarily ethylene and benzene. Ethylene selectivity increased with time for higher Mo loading and lower space velocity. Benzene product selectivity increased with higher Mo loading, lower temperature, and lower space velocity, while gradually decreasing with time. A direct experimental comparison of conventional Mo-HZSM5 synthesized here showed that the Mo-SH catalyst is more active than Mo-HZSM5.
中文翻译:

硫酸氧化f作为氧化钼的载体:甲烷脱氢芳构化的新型催化剂
甲烷催化转化是当今催化领域最具挑战性的方面之一。天然气是甲烷的丰富来源,目前主要用于发电,其中许多只是燃烧。将甲烷转化为高价值碳氢化合物的潜力巨大。除其他反应外,可通过脱氢芳构化(MDHA)将甲烷直接转化为氢气和有价值的碳氢化合物,如苯。
近年来,对于MDHA,对ZSM-5 / MCM-22负载的氧化钼进行了广泛的研究。据报道,Mo碳化物通过形成CHx物质来活化甲烷。这些被二聚为C 2 H y然后在强ZSM-5 / MCM-22布朗斯台德酸位上低聚形成苯。相关工作表明,硫酸氧化锆(SZ)为在MDHA中生产苯所需的Mo提供了酸性位点[1]。硫酸化氧化f(SH)与硫酸化氧化锆的相似性是对MDHA的合乎逻辑的新颖支持。尽管SH已用于其他酸催化反应,但尽管有这种催化剂的吸引力,但我们仍未对Mo-SH用于MDHA反应进行系统的研究。在这里,已使用SEM-EDS,XPS,XANES,DRIFTS,HR-TEM,BET和温度编程技术对Mo / SH催化剂进行了测试和表征。DRIFTS证实,向催化剂中添加Mo不会影响SH酸度。SEM-EDS和XPS已用于表征SH载体上Mo和硫的负载量。XANES以及HR-TEM分析用于研究碳化钼/碳氧化物物种的形成,这些碳化钼/碳氧化物通常被认为是MDHA的活性位点。进行了MDHA试验以研究Mo负载,温度和空速的影响。通过甲烷转化率测量,5%Mo-SH比1%Mo-SH更具活性。转化率随温度升高和空速降低而增加,并随时间逐渐失活。这可以归因于催化表面焦化,随后的TPO分析证实了这一点。观察到的产物主要是乙烯和苯。乙烯选择性随时间的增加而增加,以增加Mo的负载量和降低空速。苯产物的选择性随着较高的Mo负载量,较低的温度和较低的空速而增加,而随时间逐渐降低。



























 京公网安备 11010802027423号
京公网安备 11010802027423号