Catalysis Communications ( IF 3.7 ) Pub Date : 2019-02-10 , DOI: 10.1016/j.catcom.2019.01.015 Xin Ge , Lin Cheng , Fengli Sun , Xuemin Liu , Xinzhi Chen , Chao Qian , Shaodong Zhou
|
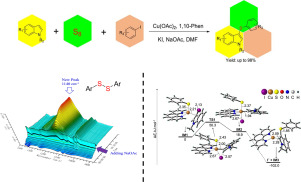
The copper-catalyzed sulfenylation of indoles with aryl iodide and sulfur powder has been investigated both experimentally and theoretically. This protocol provides a direct and facile approach to prepare 3-sulfenylindoles with moderate to excellent yields and good functional-group tolerance. The in-situ IR analysis provided evidence for that NaOAc could promote the synthesis of diphenyl disulfide by the coupling of aryl iodide and sulfur powder. According to DFT calculations, the coupling pathway involving the intermediate N-methyl-3-iodoindole is more favored than the direct coupling by the CH activation of indole. The N-methyl-3-iodoindole was identified as a crucial intermediate in the catalytic cycle.
中文翻译:

硫粉和芳基碘化物在铜上催化吲哚的C3-亚磺化反应的机理和实验研究
实验和理论研究了铜与芳基碘化物和硫粉催化的吲哚亚磺酰基化反应。该协议提供了一种直接且简便的方法来制备3-亚磺酰基吲哚,具有中等至优异的收率和良好的官能团耐受性。原位红外分析提供了证据,证明NaOAc可以通过芳基碘化物和硫粉的偶联促进二苯基二硫化物的合成。根据DFT计算,涉及中间N-甲基-3-碘吲哚的偶联途径比通过吲哚的C H活化的直接偶联更有利。所述Ñ甲基-3-碘吲哚被鉴定为在催化循环的关键中间体。


























 京公网安备 11010802027423号
京公网安备 11010802027423号