当前位置:
X-MOL 学术
›
Mol. Biosyst.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanism of the formation of the RecA–ssDNA nucleoprotein filament structure: a coarse-grained approach
Molecular BioSystems Pub Date : 2017-10-27 00:00:00 , DOI: 10.1039/c7mb00486a Goutam Mukherjee 1, 2, 3, 4 , Arumay Pal 1, 2, 3, 4 , Yaakov Levy 1, 2, 3, 4
Molecular BioSystems Pub Date : 2017-10-27 00:00:00 , DOI: 10.1039/c7mb00486a Goutam Mukherjee 1, 2, 3, 4 , Arumay Pal 1, 2, 3, 4 , Yaakov Levy 1, 2, 3, 4
Affiliation
|
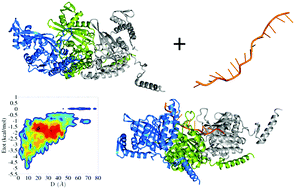
In prokaryotes, the RecA protein catalyzes the repair and strand exchange of double-stranded DNA. RecA binds to single-stranded DNA (ssDNA) and forms a presynaptic complex in which the protein polymerizes around the ssDNA to form a right-handed helical nucleoprotein filament structure. In the present work, the mechanism for the formation of the RecA–ssDNA filament structure is modeled using coarse-grained molecular dynamics simulations. Information from the X-ray structure was used to model the protein itself but not its interactions; the interactions between the protein and the ssDNA were modeled solely by electrostatic, aromatic, and repulsive energies. For the present study, the monomeric, dimeric, and trimeric units of RecA and 4, 8, and 11 NT-long ssDNA, respectively, were studied. Our results indicate that monomeric RecA is not sufficient for nucleoprotein filament formation; rather, dimeric RecA is the elementary binding unit, with higher multimeric units of RecA facilitating filament formation. Our results reveal that loop region flexibility at the primary binding site of RecA is essential for it to bind the incoming ssDNA, that the aromatic residues present in the loop region play an important role in ssDNA binding, and that ATP may play a role in guiding the ssDNA by changing the electrostatic potential of the RecA protein.
中文翻译:

RecA–ssDNA核蛋白丝结构的形成机理:一种粗粒度方法
在原核生物中,RecA蛋白催化双链DNA的修复和链交换。RecA与单链DNA(ssDNA)结合并形成突触前复合物,其中蛋白质在ssDNA周围聚合形成右旋螺旋形核蛋白丝结构。在目前的工作中,RecA–ssDNA细丝结构形成的机制是使用粗粒度的分子动力学模拟来建模的。X射线结构的信息被用来模拟蛋白质本身,而不是其相互作用。蛋白质和ssDNA之间的相互作用仅通过静电,芳香和排斥能来建模。对于本研究,分别研究了RecA和4、8和11个NT长的ssDNA的单体,二聚体和三聚体单元。我们的结果表明单体RecA不足以形成核蛋白丝。相反,二聚的RecA是基本的结合单元,RecA的较高的多聚单元有助于长丝形成。我们的研究结果表明,RecA的主要结合位点的环区柔性对于它结合传入的ssDNA至关重要,环区中存在的芳香族残基在ssDNA结合中起重要作用,而ATP可能在指导通过改变RecA蛋白的静电势来修饰ssDNA。
更新日期:2017-11-06
中文翻译:

RecA–ssDNA核蛋白丝结构的形成机理:一种粗粒度方法
在原核生物中,RecA蛋白催化双链DNA的修复和链交换。RecA与单链DNA(ssDNA)结合并形成突触前复合物,其中蛋白质在ssDNA周围聚合形成右旋螺旋形核蛋白丝结构。在目前的工作中,RecA–ssDNA细丝结构形成的机制是使用粗粒度的分子动力学模拟来建模的。X射线结构的信息被用来模拟蛋白质本身,而不是其相互作用。蛋白质和ssDNA之间的相互作用仅通过静电,芳香和排斥能来建模。对于本研究,分别研究了RecA和4、8和11个NT长的ssDNA的单体,二聚体和三聚体单元。我们的结果表明单体RecA不足以形成核蛋白丝。相反,二聚的RecA是基本的结合单元,RecA的较高的多聚单元有助于长丝形成。我们的研究结果表明,RecA的主要结合位点的环区柔性对于它结合传入的ssDNA至关重要,环区中存在的芳香族残基在ssDNA结合中起重要作用,而ATP可能在指导通过改变RecA蛋白的静电势来修饰ssDNA。



























 京公网安备 11010802027423号
京公网安备 11010802027423号