当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Halo-Jacobsen Rearrangement Induced by Steric Repulsion between peri-Iodo Groups
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2023-06-02 , DOI: 10.1021/acs.joc.3c00165 Kento Iwai 1, 2 , Noa Nishiguchi 1 , Nagatoshi Nishiwaki 1, 2
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2023-06-02 , DOI: 10.1021/acs.joc.3c00165 Kento Iwai 1, 2 , Noa Nishiguchi 1 , Nagatoshi Nishiwaki 1, 2
Affiliation
|
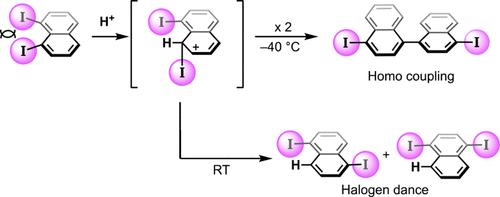
The naphthalene ring is distorted due to steric repulsion between iodo groups at the peri-positions. Due to the distortion, 1,8-diiodonaphthalene underwent a halo-Jacobsen rearrangement when treated with trifluoromethanesulfonic acid, producing 1,5-diiodonaphthalene and 1,4-diiodonaphthalene. In this reaction, acid-induced dehalogenative homocoupling also proceeded to form 4,4′-diiodo-1,1′-binaphthyl. The reaction selectivity could be controlled by varying the reaction temperature. DFT calculations and some control experiments revealed that these compounds were formed by different pathways.
中文翻译:

碘周围基团之间的空间排斥引起的晕-雅各布森重排
由于邻位碘基之间的空间排斥,萘环发生扭曲。由于畸变,1,8-二碘萘在用三氟甲磺酸处理时发生卤代-雅可布森重排,产生1,5-二碘萘和1,4-二碘萘。在此反应中,酸诱导的脱卤自偶联也继续形成 4,4'-二碘-1,1'-联萘。反应选择性可以通过改变反应温度来控制。DFT 计算和一些对照实验表明这些化合物是通过不同的途径形成的。
更新日期:2023-06-02
中文翻译:

碘周围基团之间的空间排斥引起的晕-雅各布森重排
由于邻位碘基之间的空间排斥,萘环发生扭曲。由于畸变,1,8-二碘萘在用三氟甲磺酸处理时发生卤代-雅可布森重排,产生1,5-二碘萘和1,4-二碘萘。在此反应中,酸诱导的脱卤自偶联也继续形成 4,4'-二碘-1,1'-联萘。反应选择性可以通过改变反应温度来控制。DFT 计算和一些对照实验表明这些化合物是通过不同的途径形成的。

























 京公网安备 11010802027423号
京公网安备 11010802027423号