当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Winning Combination of Cu and Fe Oxide Clusters with an Alumina Support for Low-Temperature Catalytic Oxidation of Volatile Organic Compounds
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2023-06-02 , DOI: 10.1021/acsami.3c02705 Tadej Žumbar 1 , Iztok Arčon 2 , Petar Djinović 1 , Giuliana Aquilanti 3 , Gregor Žerjav 1 , Albin Pintar 1 , Alenka Ristić 1 , Goran Dražić 1 , Janez Volavšek 1 , Gregor Mali 1 , Margarita Popova 4 , Nataša Zabukovec Logar 1, 2 , Nataša Novak Tušar 1, 2
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2023-06-02 , DOI: 10.1021/acsami.3c02705 Tadej Žumbar 1 , Iztok Arčon 2 , Petar Djinović 1 , Giuliana Aquilanti 3 , Gregor Žerjav 1 , Albin Pintar 1 , Alenka Ristić 1 , Goran Dražić 1 , Janez Volavšek 1 , Gregor Mali 1 , Margarita Popova 4 , Nataša Zabukovec Logar 1, 2 , Nataša Novak Tušar 1, 2
Affiliation
|
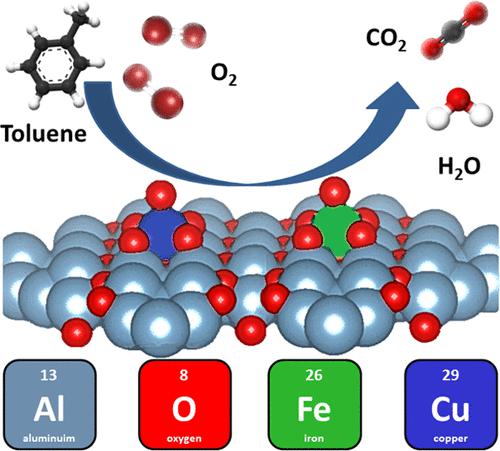
A γ-alumina support functionalized with transition metals is one of the most widely used industrial catalysts for the total oxidation of volatile organic compounds (VOCs) as air pollutants at higher temperatures (280–450 °C). By rational design of a bimetal CuFe-γ-alumina catalyst, synthesized from a dawsonite alumina precursor, the activity in total oxidation of toluene as a model VOC at a lower temperature (200–380 °C) is achieved. A fundamental understanding of the catalyst and the reaction mechanism is elucidated by advanced microscopic and spectroscopic characterizations as well as by temperature-programmed surface techniques. The nature of the metal–support bonding and the optimal abundance between Cu–O–Al and Fe–O–Al species in the catalysts leads to synergistic catalytic activity promoted by small amounts of iron (Fe/Al = 0.005). The change in the metal oxide–cluster alumina interface is related to the nature of the surfaces to which the Cu atoms attach. In the most active catalyst, the CuO6 octahedra are attached to 4 Al atoms, while in the less active catalyst, they are attached to only 3 Al atoms. The oxidation of toluene occurs via the Langmuir–Hinshelwood mechanism. The presented material introduces a prospective family of low-cost and scalable oxidation catalysts with superior efficiency at lower temperatures.
中文翻译:

Cu 和 Fe 氧化物团簇与氧化铝载体的成功组合用于挥发性有机化合物的低温催化氧化
用过渡金属功能化的γ-氧化铝载体是最广泛使用的工业催化剂之一,用于在较高温度(280-450°C)下将挥发性有机化合物(VOC)作为空气污染物完全氧化。通过合理设计由片钠铝石氧化铝前体合成的双金属 CuFe-γ-氧化铝催化剂,实现了在较低温度(200-380 °C)下作为模型 VOC 的甲苯全氧化活性。通过先进的显微和光谱表征以及程序升温表面技术阐明了对催化剂和反应机理的基本理解。金属-载体键合的性质以及催化剂中 Cu-O-Al 和 Fe-O-Al 物种之间的最佳丰度导致少量铁 (Fe/Al = 0.005) 促进的协同催化活性。金属氧化物-团簇氧化铝界面的变化与铜原子附着的表面性质有关。在活性最强的催化剂中,CuO6 个八面体与 4 个铝原子相连,而在活性较低的催化剂中,它们仅与 3 个铝原子相连。甲苯的氧化通过 Langmuir-Hinshelwood 机制发生。所展示的材料介绍了一系列具有前景的低成本且可扩展的氧化催化剂,它们在较低温度下具有出色的效率。
更新日期:2023-06-02
中文翻译:

Cu 和 Fe 氧化物团簇与氧化铝载体的成功组合用于挥发性有机化合物的低温催化氧化
用过渡金属功能化的γ-氧化铝载体是最广泛使用的工业催化剂之一,用于在较高温度(280-450°C)下将挥发性有机化合物(VOC)作为空气污染物完全氧化。通过合理设计由片钠铝石氧化铝前体合成的双金属 CuFe-γ-氧化铝催化剂,实现了在较低温度(200-380 °C)下作为模型 VOC 的甲苯全氧化活性。通过先进的显微和光谱表征以及程序升温表面技术阐明了对催化剂和反应机理的基本理解。金属-载体键合的性质以及催化剂中 Cu-O-Al 和 Fe-O-Al 物种之间的最佳丰度导致少量铁 (Fe/Al = 0.005) 促进的协同催化活性。金属氧化物-团簇氧化铝界面的变化与铜原子附着的表面性质有关。在活性最强的催化剂中,CuO6 个八面体与 4 个铝原子相连,而在活性较低的催化剂中,它们仅与 3 个铝原子相连。甲苯的氧化通过 Langmuir-Hinshelwood 机制发生。所展示的材料介绍了一系列具有前景的低成本且可扩展的氧化催化剂,它们在较低温度下具有出色的效率。



























 京公网安备 11010802027423号
京公网安备 11010802027423号