当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Functionalization of Pyridine and Quinoline Scaffolds by Using Organometallic Li-, Mg- and Zn-Reagents
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2023-06-01 , DOI: 10.1002/ejoc.202300285 Vasudevan Dhayalan 1 , Deepika Sharma 2 , Rana Chatterjee Chatterjee 2 , Rambabu Dandela 2
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2023-06-01 , DOI: 10.1002/ejoc.202300285 Vasudevan Dhayalan 1 , Deepika Sharma 2 , Rana Chatterjee Chatterjee 2 , Rambabu Dandela 2
Affiliation
|
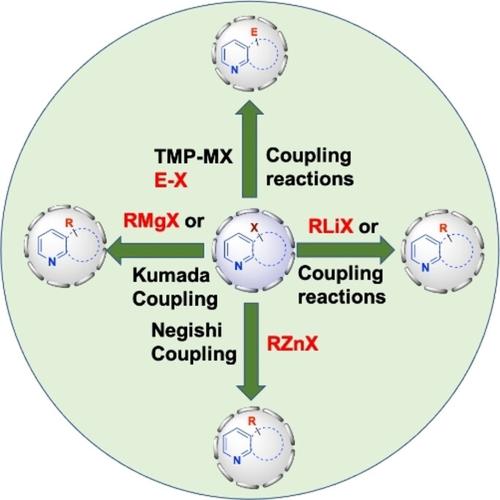
The different approaches to functionalizing pyridine and quinoline scaffolds are covered in this review, including direct selective metalation (DoM), halogen/metal exchange reactions, Li, Mg, and Zn insertion, and trans-metalation approaches, which are then followed by cross-coupling reactions of the Kumada or Negishi types. Sensitive maybe broad functional group tolerance, moderate and sustainable reaction conditions, and inexpensive and low-hazardous chemicals were used to highly functionalize pyridine and quinoline-based bioactive pharmaceutical scaffolds and natural products.
中文翻译:

使用有机金属锂、镁和锌试剂对吡啶和喹啉支架进行官能化
本综述涵盖了吡啶和喹啉支架功能化的不同方法,包括直接选择性金属化(DoM)、卤素/金属交换反应、Li、Mg和Zn插入以及转金属化方法,然后是交叉金属化方法。 Kumada 或 Negishi 类型的偶联反应。敏感的可能广泛的官能团耐受性、温和且可持续的反应条件以及廉价且低危害的化学品被用来对基于吡啶和喹啉的生物活性药物支架和天然产物进行高度功能化。
更新日期:2023-06-01
中文翻译:

使用有机金属锂、镁和锌试剂对吡啶和喹啉支架进行官能化
本综述涵盖了吡啶和喹啉支架功能化的不同方法,包括直接选择性金属化(DoM)、卤素/金属交换反应、Li、Mg和Zn插入以及转金属化方法,然后是交叉金属化方法。 Kumada 或 Negishi 类型的偶联反应。敏感的可能广泛的官能团耐受性、温和且可持续的反应条件以及廉价且低危害的化学品被用来对基于吡啶和喹啉的生物活性药物支架和天然产物进行高度功能化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号