当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Methane Activation by [OsC3]+: Implications for Catalyst Design
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2023-06-01 , DOI: 10.1021/acs.jpclett.3c00982 Shihan Li 1 , Xiao-Nan Wu 2 , Shaodong Zhou 1, 3
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2023-06-01 , DOI: 10.1021/acs.jpclett.3c00982 Shihan Li 1 , Xiao-Nan Wu 2 , Shaodong Zhou 1, 3
Affiliation
|
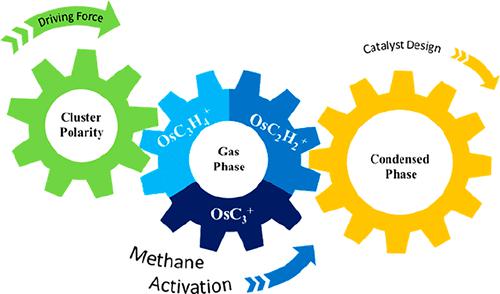
Gas-phase reactions of [OsC3]+ with methane at ambient temperature have been studied by using quadrupole-ion trap mass spectrometry combined with quantum chemical calculations. The comparison of [OsC3]+ with the product clusters revealed significant changes in cluster reactivity. In particular, with different ligands, the cluster may produce multiple products or, alternatively, just a single product. Theoretical calculations reveal the influence of electronic features such as molecular polarity index, charge and spin distribution, and HOMO–LUMO gap on the reactivity of the Os complexes. Fundamentally, it is the polarity of the clusters that leads to the cluster reactivity in the methane activation. Furthermore, reducing the local polarity of the catalyst active site may be one means of reducing the number of byproducts in the reaction.
中文翻译:

[OsC3]+ 激活甲烷:对催化剂设计的影响
利用四极离子阱质谱结合量子化学计算研究了常温下[OsC 3 ] +与甲烷的气相反应。[OsC 3 ] +的比较与产品集群揭示了集群反应性的显着变化。特别是,对于不同的配体,簇可能会产生多种产物,或者只产生一种产物。理论计算揭示了分子极性指数、电荷和自旋分布以及 HOMO-LUMO 间隙等电子特征对 Os 配合物反应性的影响。从根本上说,正是团簇的极性导致了甲烷活化中的团簇反应性。此外,降低催化剂活性位点的局部极性可能是减少反应中副产物数量的一种手段。
更新日期:2023-06-01
中文翻译:

[OsC3]+ 激活甲烷:对催化剂设计的影响
利用四极离子阱质谱结合量子化学计算研究了常温下[OsC 3 ] +与甲烷的气相反应。[OsC 3 ] +的比较与产品集群揭示了集群反应性的显着变化。特别是,对于不同的配体,簇可能会产生多种产物,或者只产生一种产物。理论计算揭示了分子极性指数、电荷和自旋分布以及 HOMO-LUMO 间隙等电子特征对 Os 配合物反应性的影响。从根本上说,正是团簇的极性导致了甲烷活化中的团簇反应性。此外,降低催化剂活性位点的局部极性可能是减少反应中副产物数量的一种手段。


























 京公网安备 11010802027423号
京公网安备 11010802027423号