当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
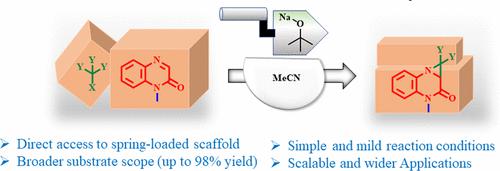
Direct Access to Strained Fused Dihalo-Aziridino Quinoxalinones via C3-Alkylation Followed by Tandem Cyclization
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2023-06-01 , DOI: 10.1021/acs.joc.3c00033 Vikas V Khade 1 , Anindita Bhowmick 1 , Archana S Thube 1 , Ramakrishna G Bhat 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2023-06-01 , DOI: 10.1021/acs.joc.3c00033 Vikas V Khade 1 , Anindita Bhowmick 1 , Archana S Thube 1 , Ramakrishna G Bhat 1
Affiliation
|
Quinoxalinones are a privileged class of compounds, and their structural framework is found in many bioactive compounds, natural compounds, and pharmaceuticals. Quinoxalinone is a promising scaffold for different types of functionalization, and the slight modification of the quinoxalinone skeleton is known to offer a wide range of compounds for drug discovery. Owing to the importance of the quinoxalinone scaffold, we have developed a base-mediated protocol for the C3-alkylation of quinoxalinone followed by tandem cyclization to access novel types of strenuous and fused dihalo-aziridino-quinoxalinone heterocycles via the construction of C–C and C–N bonds. The protocol proved to be simple and practical to access desired fused quinoxalinone heterocycles in excellent yields (up to 98% yield). As an application, the highly functionalized fused dihalo-aziridino-quinoxalinone molecule has been further utilized for mono-dehalogenation under visible light irradiation and selective amide reduction. Moreover, the protocol has also been demonstrated on a gram scale.
中文翻译:

通过 C3-烷基化和随后的串联环化直接获得应变稠合二卤代氮丙啶基喹喔啉酮
喹喔啉酮是一类特殊的化合物,其结构框架存在于许多生物活性化合物、天然化合物和药物中。喹喔啉酮是一种有前途的用于不同类型功能化的支架,并且已知对喹喔啉酮骨架的轻微修饰可以为药物发现提供广泛的化合物。由于喹喔啉酮支架的重要性,我们开发了一种碱基介导的喹喔啉酮 C3 烷基化方案,然后串联环化,通过构建 C-C 和C-N 键。事实证明,该方案简单实用,能够以优异的产率(高达 98% 的产率)获得所需的稠合喹喔啉酮杂环。作为一个应用程序,高度功能化的稠合二卤代氮丙啶基喹喔啉酮分子已进一步用于可见光照射下的单脱卤和选择性酰胺还原。此外,该协议还在克规模上进行了演示。
更新日期:2023-06-01
中文翻译:

通过 C3-烷基化和随后的串联环化直接获得应变稠合二卤代氮丙啶基喹喔啉酮
喹喔啉酮是一类特殊的化合物,其结构框架存在于许多生物活性化合物、天然化合物和药物中。喹喔啉酮是一种有前途的用于不同类型功能化的支架,并且已知对喹喔啉酮骨架的轻微修饰可以为药物发现提供广泛的化合物。由于喹喔啉酮支架的重要性,我们开发了一种碱基介导的喹喔啉酮 C3 烷基化方案,然后串联环化,通过构建 C-C 和C-N 键。事实证明,该方案简单实用,能够以优异的产率(高达 98% 的产率)获得所需的稠合喹喔啉酮杂环。作为一个应用程序,高度功能化的稠合二卤代氮丙啶基喹喔啉酮分子已进一步用于可见光照射下的单脱卤和选择性酰胺还原。此外,该协议还在克规模上进行了演示。



























 京公网安备 11010802027423号
京公网安备 11010802027423号