当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modeling Uranyl Adsorption on MoS2/Mo2CTx Heterostructures Using DFT and BOMD Methods
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2023-06-01 , DOI: 10.1021/acs.inorgchem.3c00625 Cheng Meng 1, 2 , Weihui Shu 1 , Kun Zhao 3 , Chunpei Yan 1, 2 , Zuojia Li 1, 2 , Qianglin Wei 2 , Minjia Wang 4 , Yunhai Liu 1, 2 , Zhibin Zhang 1, 2
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2023-06-01 , DOI: 10.1021/acs.inorgchem.3c00625 Cheng Meng 1, 2 , Weihui Shu 1 , Kun Zhao 3 , Chunpei Yan 1, 2 , Zuojia Li 1, 2 , Qianglin Wei 2 , Minjia Wang 4 , Yunhai Liu 1, 2 , Zhibin Zhang 1, 2
Affiliation
|
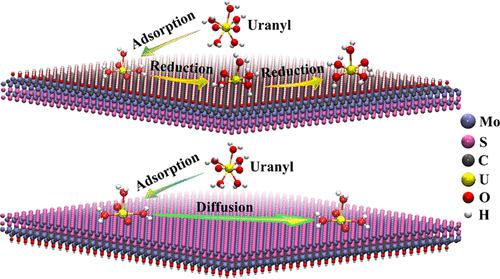
Uranium-bearing wastewaters exert a great threat to the ecological environment due to its high radiotoxicity level. The designing and fabrication of novel adsorption materials can be promoted for radionuclide elimination from wastewater. In this work, results from density functional theory and Born–Oppenheimer molecular dynamics simulations are reported for the uranyl adsorption behavior on the MoS2/Mo2CTx heterostructure in the gas phase and in an aqueous environment. Uranyl ions prefer to be adsorbed at deprotonated O sites on the Mo2COH surface and S sites on the MoS2 side of the heterojunctions, resulting in the formation of bidentate configurations. In addition to coordination interaction, H-bond and van der Waals interactions can also play an important role in binding configurations. More importantly, the oxidation state U(VI) can be reduced to U(V) and then to U(IV) caused by the strong reducibility of the Mo2COH surface at room temperature, whereas the uranyl complex can move freely on the MoS2 surface. However, the coordination number of U with respect to H2O in the first hydration shell on the Mo2COH surface remains unchanged and is found to be 3, which is similar to that on the MoS2 surface. This work provides novel nanosorbents for the removal of uranyl from wastewater. The present viewpoint provides valuable mechanistic interpretations for uranyl adsorption and will give a supplement to the experimental research.
中文翻译:

使用 DFT 和 BOMD 方法模拟铀酰在 MoS2/Mo2CTx 异质结构上的吸附
含铀废水由于其高放射毒性水平,对生态环境造成了巨大威胁。新型吸附材料的设计和制造可以促进废水中放射性核素的去除。在这项工作中,报告了密度泛函理论和 Born-Oppenheimer 分子动力学模拟的结果,以了解气相和水环境中铀酰在 MoS 2 /Mo 2 CT x异质结构上的吸附行为。铀酰离子更倾向于吸附在 Mo 2 COH 表面的去质子化 O 位点和 MoS 2表面的 S 位点异质结的一侧,导致双齿配置的形成。除了配位相互作用,氢键和范德华力相互作用也可以在结合构型中发挥重要作用。更重要的是,由于 Mo 2 COH 表面在室温下的强还原性,氧化态 U(VI) 可以还原为 U(V),然后还原为 U(IV),而铀酰络合物可以在 MoS 上自由移动2表面。然而,Mo 2 COH表面第一水合壳层中U相对于H 2 O的配位数保持不变,为3,这与MoS 2上的相近表面。这项工作为去除废水中的铀酰提供了新型纳米吸附剂。目前的观点为铀酰吸附提供了有价值的机理解释,并将为实验研究提供补充。
更新日期:2023-06-01
中文翻译:

使用 DFT 和 BOMD 方法模拟铀酰在 MoS2/Mo2CTx 异质结构上的吸附
含铀废水由于其高放射毒性水平,对生态环境造成了巨大威胁。新型吸附材料的设计和制造可以促进废水中放射性核素的去除。在这项工作中,报告了密度泛函理论和 Born-Oppenheimer 分子动力学模拟的结果,以了解气相和水环境中铀酰在 MoS 2 /Mo 2 CT x异质结构上的吸附行为。铀酰离子更倾向于吸附在 Mo 2 COH 表面的去质子化 O 位点和 MoS 2表面的 S 位点异质结的一侧,导致双齿配置的形成。除了配位相互作用,氢键和范德华力相互作用也可以在结合构型中发挥重要作用。更重要的是,由于 Mo 2 COH 表面在室温下的强还原性,氧化态 U(VI) 可以还原为 U(V),然后还原为 U(IV),而铀酰络合物可以在 MoS 上自由移动2表面。然而,Mo 2 COH表面第一水合壳层中U相对于H 2 O的配位数保持不变,为3,这与MoS 2上的相近表面。这项工作为去除废水中的铀酰提供了新型纳米吸附剂。目前的观点为铀酰吸附提供了有价值的机理解释,并将为实验研究提供补充。



























 京公网安备 11010802027423号
京公网安备 11010802027423号