当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Formation of Heterobimetallic Complexes by Addition of d10-Metal Ions to [(Me3P)xM(2-C6F4PPh2)2] (x = 1, 2; M = Ni and Pt): A Synthetic and Computational Study of Metallophilic Interactions
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2023-05-31 , DOI: 10.1021/acs.inorgchem.3c00311 Robert Gericke 1, 2 , Martin A Bennett 3 , Steven H Privér 1 , Suresh K Bhargava 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2023-05-31 , DOI: 10.1021/acs.inorgchem.3c00311 Robert Gericke 1, 2 , Martin A Bennett 3 , Steven H Privér 1 , Suresh K Bhargava 1
Affiliation
|
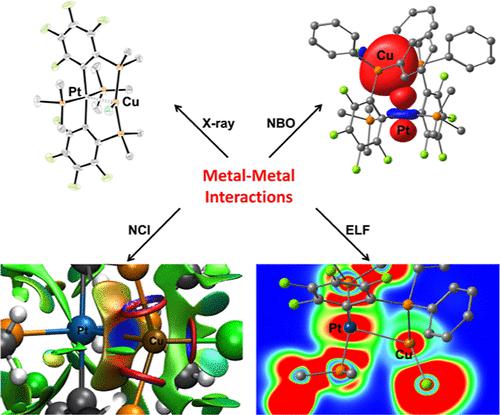
Treatment of the bis(chelate) complexes trans-[M(κ2-2-C6F4PPh2)2] (trans-1M; M = Ni, Pt) and cis-[Pt(κ2-2-C6F4PPh2)2] (cis-1Pt) with equimolar amounts or excess of PMe3 solution gave complexes of the type [(Me3P)xM(2-C6F4PPh2)2] (x = 2: 2Ma, 2Mb x = 1: 3Ma, 3Mb; M = Ni, Pt). The reactivity of complexes of the type 2M and 3M toward monovalent coinage metal ions (M′ = Cu, Ag, Au) was investigated next to the reaction of 1M toward [AuCl(PMe3)]. Four different complex types [(Me3P)2M(μ-2-C6F4PPh2)2M′Cl] (5MM′; M = Ni, Pt; M′ = Cu, Ag, Au), [(Me3P)M(κ2-2-C6F4PPh2)(μ-2-C6F4PPh2)M′Cl]x (x = 1: 6MM′; M = Pt; M′ = Cu, Au; x = 2: 6PtAg), head-to-tail-[(Me3P)ClM(μ-2-C6F4PPh2)2M′] (7MM′; M = Ni, Pt; M′ = Au), and head-to-head-[(Me3P)ClM(μ-2-C6F4PPh2)2M′] (8MM′; M = Ni, Pt; M′ = Cu, Ag, Au) were observed. Single-crystal X-ray analyses of complexes 5–8 revealed short metal–metal separations (2.7124(3)–3.3287(7) Å), suggestive of attractive metal–metal interactions. Quantum chemical calculations (atoms in molecules (AIM), electron localization function (ELF), non-covalent interaction (NCI), and natural bond orbital (NBO)) gave theoretical support that the interaction characteristics reach from a pure attractive non-covalent to an electron-shared (covalent) character.
更新日期:2023-05-31


























 京公网安备 11010802027423号
京公网安备 11010802027423号