当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Constructing Type-II and S-Scheme Heterojunctions of Cu2O@Cu4(SO4)(OH)6·H2O Polyhedra by In Situ Etching Cu2O with Different Exposed Facets for Enhanced Photocatalytic Sterilization and Degradation Performance
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2023-05-31 , DOI: 10.1021/acs.inorgchem.3c01220 Jin Du , Yongjian Hu , Xia Wan , Shaolong Tie , Sheng Lan , Xingsen Gao
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2023-05-31 , DOI: 10.1021/acs.inorgchem.3c01220 Jin Du , Yongjian Hu , Xia Wan , Shaolong Tie , Sheng Lan , Xingsen Gao
|
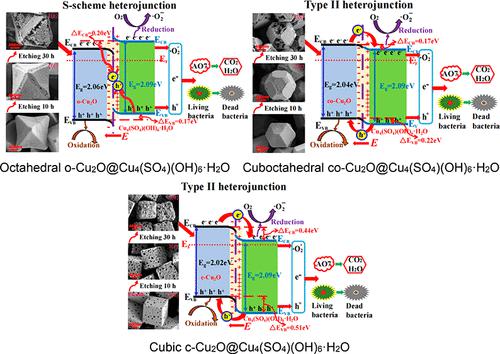
The construction of type-II or S-scheme heterojunctions can effectively accelerate the directional migration of charge carriers and inhibit the recombination of electron–hole pairs to improve the catalytic performance of the composite catalyst; therefore, the construction and formation mechanism of a heterojunction are worth further investigation. Herein, Cu2O@Cu4(SO4)(OH)6·H2O core–shell polyhedral heterojunctions were fabricated via in situ etching Cu2O with octahedral, cuboctahedral, and cubic shapes by sodium thiosulfate (Na2S2O3). Cu2O@Cu4(SO4)(OH)6·H2O polyhedral heterojunctions demonstrated obviously enhanced sterilization and degradation performance than the corresponding single Cu2O polyhedra and Cu4(SO4)(OH)6·H2O. When Cu2O with a different morphology contacts with Cu4(SO4)(OH)6·H2O, a built-in electric field is established at the interface due to the difference in Fermi level (Ef); meanwhile, the direction of band bending and the band alignment are determined. These lead to the different migration pathways of electrons and holes, and thereby, a type-II or S-scheme heterojunction is constructed. The results showed that octahedral o-Cu2O@Cu4(SO4)(OH)6·H2O is an S-scheme heterojunction; however, cuboctahedral co-Cu2O@Cu4(SO4)(OH)6·H2O and cubic c-Cu2O@Cu4(SO4)(OH)6·H2O are type-II heterojunctions. By means of X-ray photoelectron spectroscopy (XPS), ultraviolet photoelectron spectroscopy (UPS), diffuse reflectance spectra (DRS), and Mott–Schottky analyses, the band alignments, Fermi levels, and band offsets (ΔECB, ΔEVB) of Cu2O@Cu4(SO4)(OH)6·H2O polyhedral heterojunctions were estimated; the results indicated that the catalytic ability of the composite catalyst is determined by the type of heterojunction and the sizes of band offsets. Cubic c-Cu2O@Cu4(SO4)(OH)6·H2O has the strongest driving force (namely, biggest band offsets) to accelerate charge migration and effectively separate charge carriers, so it exhibits the strongest catalytic bactericidal and degrading abilities.
中文翻译:

通过原位蚀刻具有不同暴露面的 Cu2O 构建 Cu2O@Cu4(SO4)(OH)6·H2O 多面体的 II 型和 S 型异质结,以增强光催化杀菌和降解性能
II型或S型异质结的构建可有效加速载流子的定向迁移,抑制电子-空穴对的复合,提高复合催化剂的催化性能;因此,异质结的构建和形成机制值得进一步研究。在此,采用硫代硫酸钠(Na 2 S 2 _ _ _ _ _ O 3)。Cu 2 O@Cu 4 (SO4 )(OH) 6 ·H 2 O多面体异质结比相应的单一Cu 2 O多面体和Cu 4 (SO 4 )(OH) 6 ·H 2 O具有明显增强的杀菌和降解性能。当Cu 2 O与不同的形貌与Cu 4 (SO 4 )(OH) 6 ·H 2 O接触,由于费米能级( E f )的差异在界面处建立了内建电场); 同时,确定了带弯曲的方向和带排列。这些导致电子和空穴的不同迁移路径,从而构建II型或S型异质结。结果表明,八面体o-Cu 2 O@Cu 4 (SO 4 )(OH) 6 ·H 2 O为S型异质结;然而,立方八面体co-Cu 2 O@Cu 4 (SO 4 )(OH) 6 ·H 2 O和立方c-Cu 2 O@Cu 4 (SO 4 )(OH) 6 ·H 2O是II型异质结。通过 X 射线光电子能谱 (XPS)、紫外光电子能谱 (UPS)、漫反射光谱 (DRS) 和 Mott–Schottky 分析,能带排列、费米能级和能带偏移 (Δ E CB , Δ E VB )的Cu 2 O@Cu 4 (SO 4 )(OH) 6 ·H 2 O多面体异质结被估计;结果表明,复合催化剂的催化能力取决于异质结的类型和带偏移的大小。立方c-Cu 2 O@Cu 4 (SO 4 )(OH) 6 ·H 2O 具有最强的驱动力(即最大的带偏移)来加速电荷迁移并有效分离电荷载流子,因此它表现出最强的催化杀菌和降解能力。
更新日期:2023-05-31
中文翻译:

通过原位蚀刻具有不同暴露面的 Cu2O 构建 Cu2O@Cu4(SO4)(OH)6·H2O 多面体的 II 型和 S 型异质结,以增强光催化杀菌和降解性能
II型或S型异质结的构建可有效加速载流子的定向迁移,抑制电子-空穴对的复合,提高复合催化剂的催化性能;因此,异质结的构建和形成机制值得进一步研究。在此,采用硫代硫酸钠(Na 2 S 2 _ _ _ _ _ O 3)。Cu 2 O@Cu 4 (SO4 )(OH) 6 ·H 2 O多面体异质结比相应的单一Cu 2 O多面体和Cu 4 (SO 4 )(OH) 6 ·H 2 O具有明显增强的杀菌和降解性能。当Cu 2 O与不同的形貌与Cu 4 (SO 4 )(OH) 6 ·H 2 O接触,由于费米能级( E f )的差异在界面处建立了内建电场); 同时,确定了带弯曲的方向和带排列。这些导致电子和空穴的不同迁移路径,从而构建II型或S型异质结。结果表明,八面体o-Cu 2 O@Cu 4 (SO 4 )(OH) 6 ·H 2 O为S型异质结;然而,立方八面体co-Cu 2 O@Cu 4 (SO 4 )(OH) 6 ·H 2 O和立方c-Cu 2 O@Cu 4 (SO 4 )(OH) 6 ·H 2O是II型异质结。通过 X 射线光电子能谱 (XPS)、紫外光电子能谱 (UPS)、漫反射光谱 (DRS) 和 Mott–Schottky 分析,能带排列、费米能级和能带偏移 (Δ E CB , Δ E VB )的Cu 2 O@Cu 4 (SO 4 )(OH) 6 ·H 2 O多面体异质结被估计;结果表明,复合催化剂的催化能力取决于异质结的类型和带偏移的大小。立方c-Cu 2 O@Cu 4 (SO 4 )(OH) 6 ·H 2O 具有最强的驱动力(即最大的带偏移)来加速电荷迁移并有效分离电荷载流子,因此它表现出最强的催化杀菌和降解能力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号